Clinical Case Reports
Point-of-care ultrasound in the diagnosis of venous thromboembolism in a rural setting
ABSTRACT: Deep vein thrombosis and pulmonary embolism can result in serious complications when diagnosis is delayed or missed. Definitive diagnosis often relies on consultative imaging, which may not be readily accessible in rural settings. Point-of-care ultrasound has been emerging as an accurate and reliable method of rapidly diagnosing deep vein thrombosis. We present a case report in which a patient presented to a rural emergency department with a chief complaint of dyspnea and was found on point-of-care ultrasound to have a right deep vein thrombosis, which resulted in the prompt recognition of a possible diagnosis of pulmonary embolism versus an asthma exacerbation. Anticoagulation was appropriately initiated prior to obtaining consultative imaging, which confirmed a right deep vein thrombosis and bilateral pulmonary emboli. This case report highlights the utility and reliability of using point-of-care ultrasound for diagnosing deep vein thrombosis in settings with limited access to immediate consultative imaging. The major barriers in improving the use of point-of-care ultrasound in rural British Columbia are a lack of integrated training and concerns about funding and availability of training courses, which highlights the need to implement formal training in medical school and family medicine residency.
Point-of-care ultrasound can be used where consultative diagnostic imaging is not readily available. When performed by trained personnel, it has high sensitivity and specificity comparable to that of duplex ultrasonography.
Venous thromboembolism encompasses both deep vein thrombosis and pulmonary embolism. The development of a deep vein thrombosis is a significant health condition that affects an estimated 49 000 Canadians annually,[1] and it can be associated with significant morbidity as well as potentially fatal complications if the diagnosis is delayed or missed. Deep vein thrombosis is characterized by the formation of thrombi in the deep venous systems, most commonly in the lower extremities. If not promptly treated, there is a 50% risk for patients with deep vein thrombosis to develop a pulmonary embolism within 3 months[2] as a consequence of dislodged thrombi that travel to the pulmonary arteries. Most pulmonary embolisms originate from a thrombus in the venous system of the lower extremities; approximately 70% of patients with symptomatic pulmonary embolism also have deep vein thrombosis.[2] Acute pulmonary embolism carries a high mortality rate of 15%; the greatest risk occurs in cases of submassive or massive pulmonary embolism, which can result in obstructive shock.[3,4] Deep vein thrombosis is also associated with a high burden of morbidity: one-third of patients develop recurrent thrombosis within 10 years,[5] and one-third develop post-thrombotic syndrome, manifesting as chronic venous insufficiency with persistent extremity pain and swelling.[6]
While the most commonly used test in diagnosing deep vein thrombosis is duplex ultrasonography,[7] point-of-care ultrasound (POCUS) is emerging as a reliable technique for the rapid diagnosis of deep vein thrombosis, especially in communities where consultative imaging services are not readily available.[8-13]
We present a case report in which the use of POCUS led to the prompt detection of a deep vein thrombosis and aided in the diagnosis of a pulmonary embolism in a rural setting. This case report illustrates the utility of using POCUS for diagnosis of deep vein thrombosis to help make informed diagnostic and management decisions, and it highlights the reliability of POCUS findings when performed by trained providers, particularly in settings with limited access to immediate consultative imaging.
Case data
A 61-year-old male tourist from British Columbia’s Interior region presented to the emergency department on Haida Gwaii with a 3-day history of dry cough and dyspnea on exertion. Haida Gwaii is a remote island archipelago 120 km off BC’s north coast and is accessed by a full-day ferry ride from Prince Rupert, the nearest referral hospital. Though no wheezing was present, he attributed his symptoms to an asthma exacerbation and requested a prescription for inhalers, because his travels to Haida Gwaii had taken him through heavy wildfire smoke. His wife noted cyanotic lips at times. Besides asthma, he reported a history of two right-leg deep vein thromboses following right-knee surgery in 2013 and prolonged travel in 2015, both of which were treated with appropriate anticoagulation without apparent sequelae. He had not received any hypercoagulability workup for his prior deep vein thromboses.
Examination showed a heart rate of 102 beats/minute, blood pressure of 134/89, and oxygen saturation of 97%, with no dyspnea at rest or increased work of breathing. Respiratory exam showed no wheeze. However, bedside spirometry demonstrated a reduced forced vital capacity, 3.32 L or 69.9% predicted, a reduced forced expiratory volume in 1 second, 2.36 L or 65.8% predicted. Post-bronchodilator forced expiratory volume in 1 second improved modestly to 2.72 L or 15.0%. Examination of the lower extremities showed no swelling, edema, or tenderness to palpation in any location.
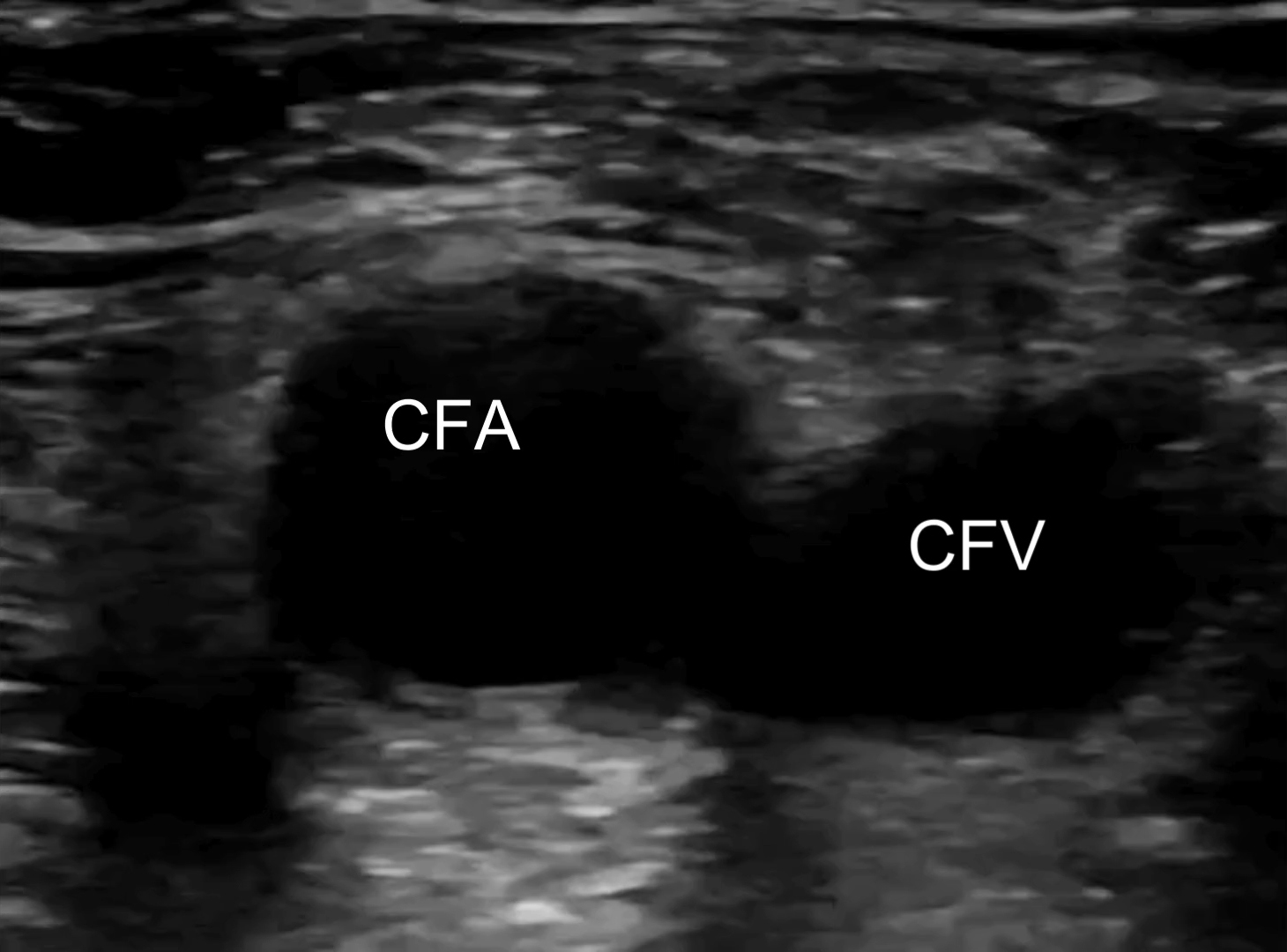
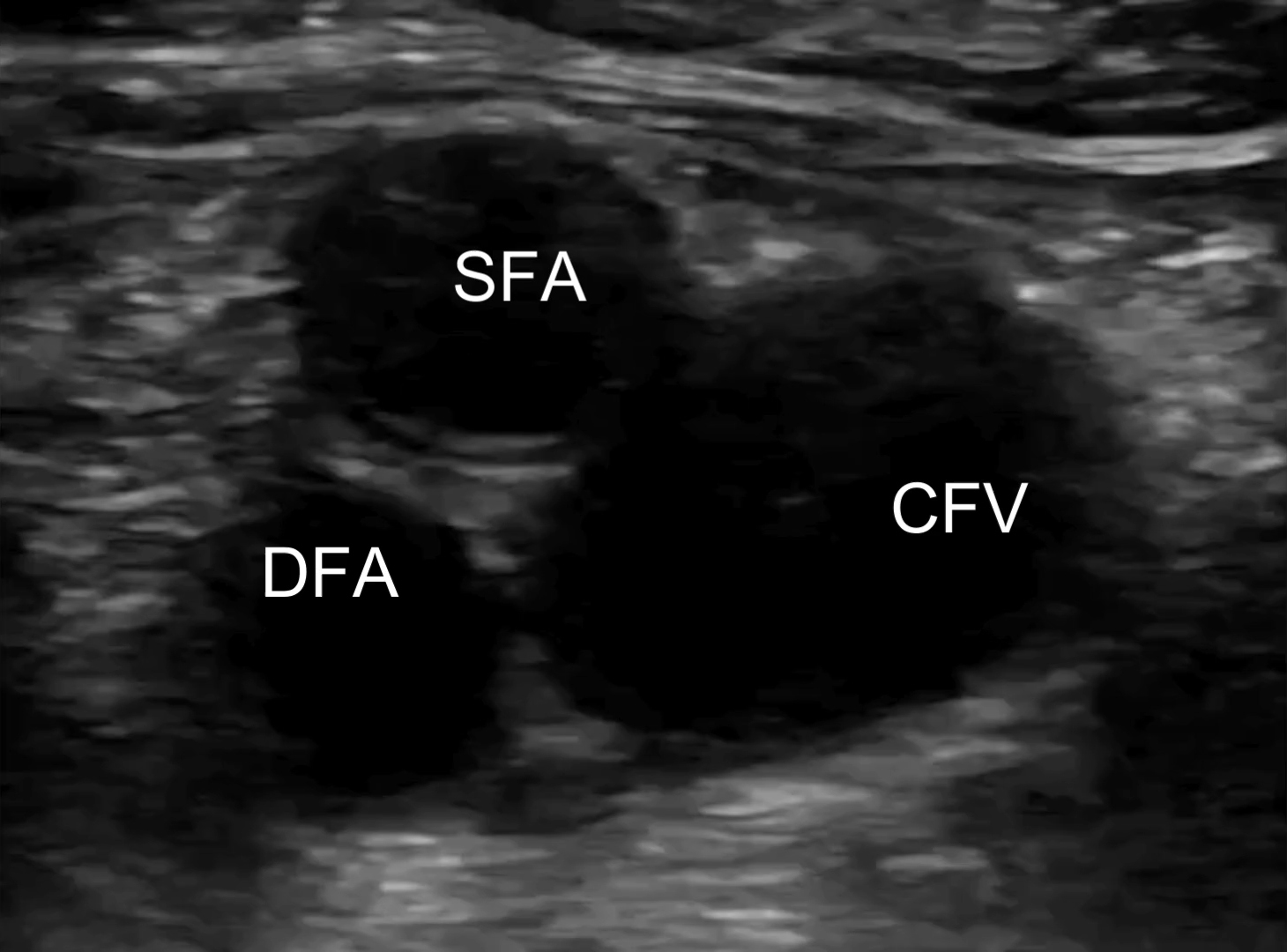
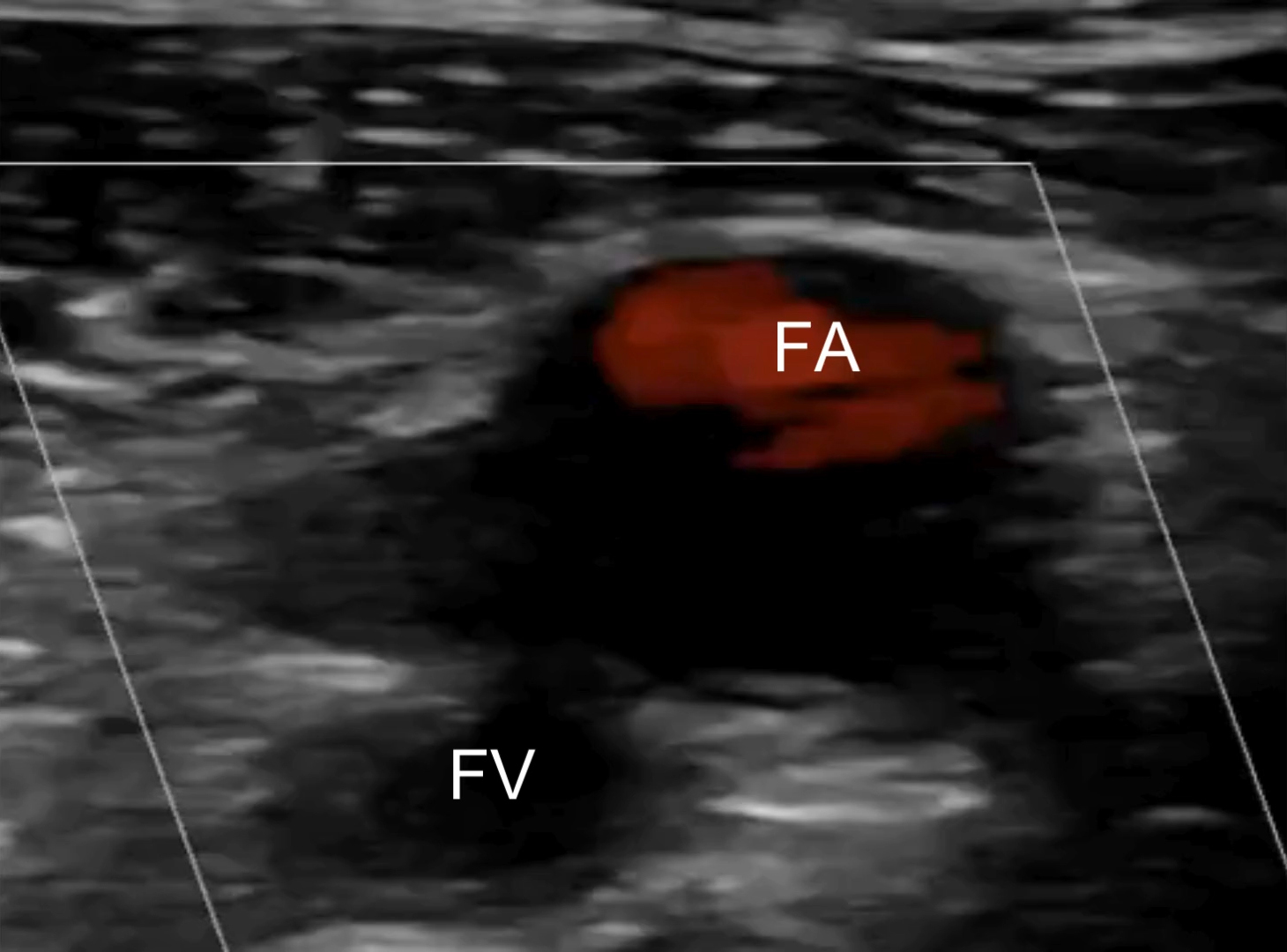
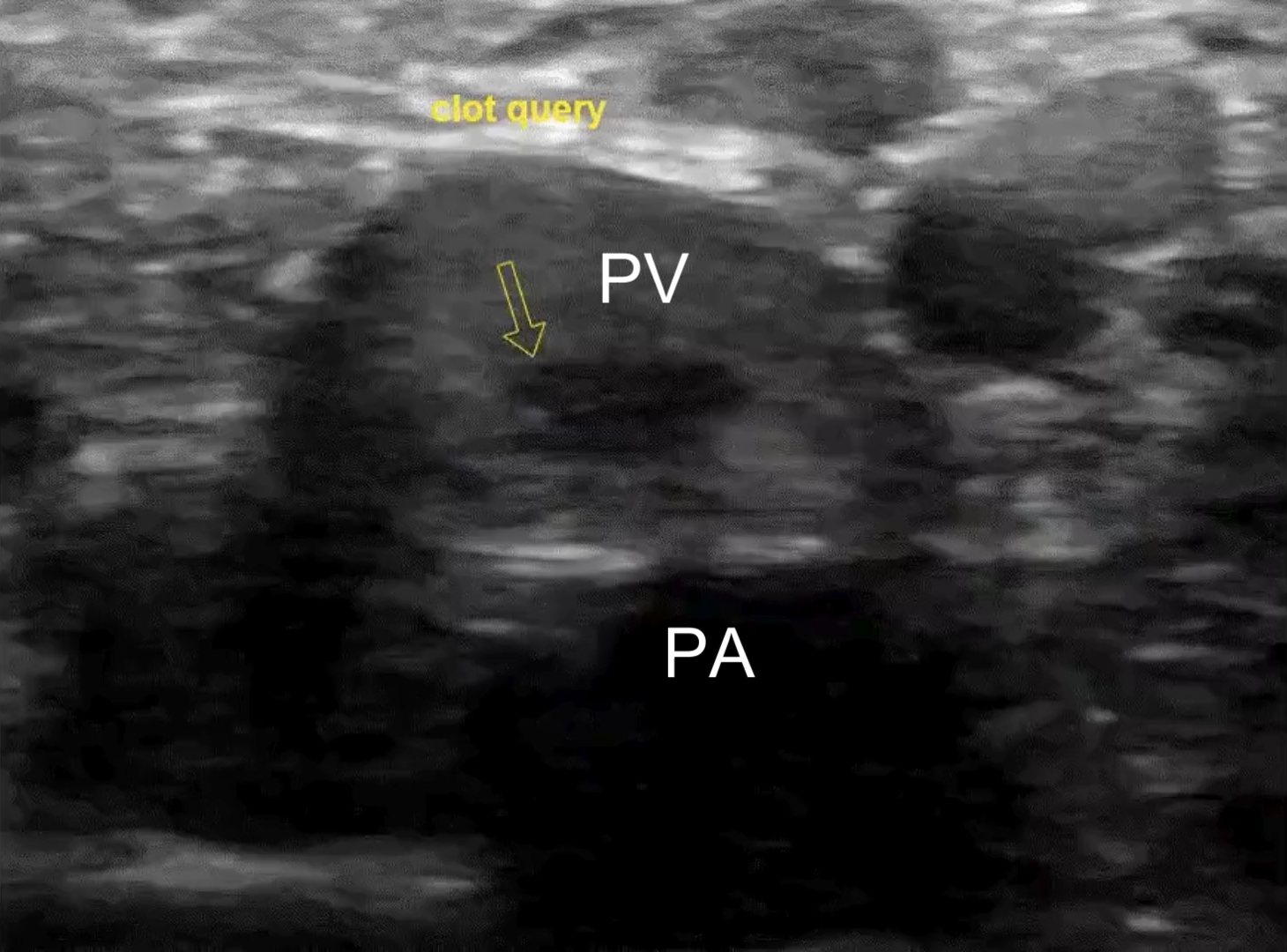
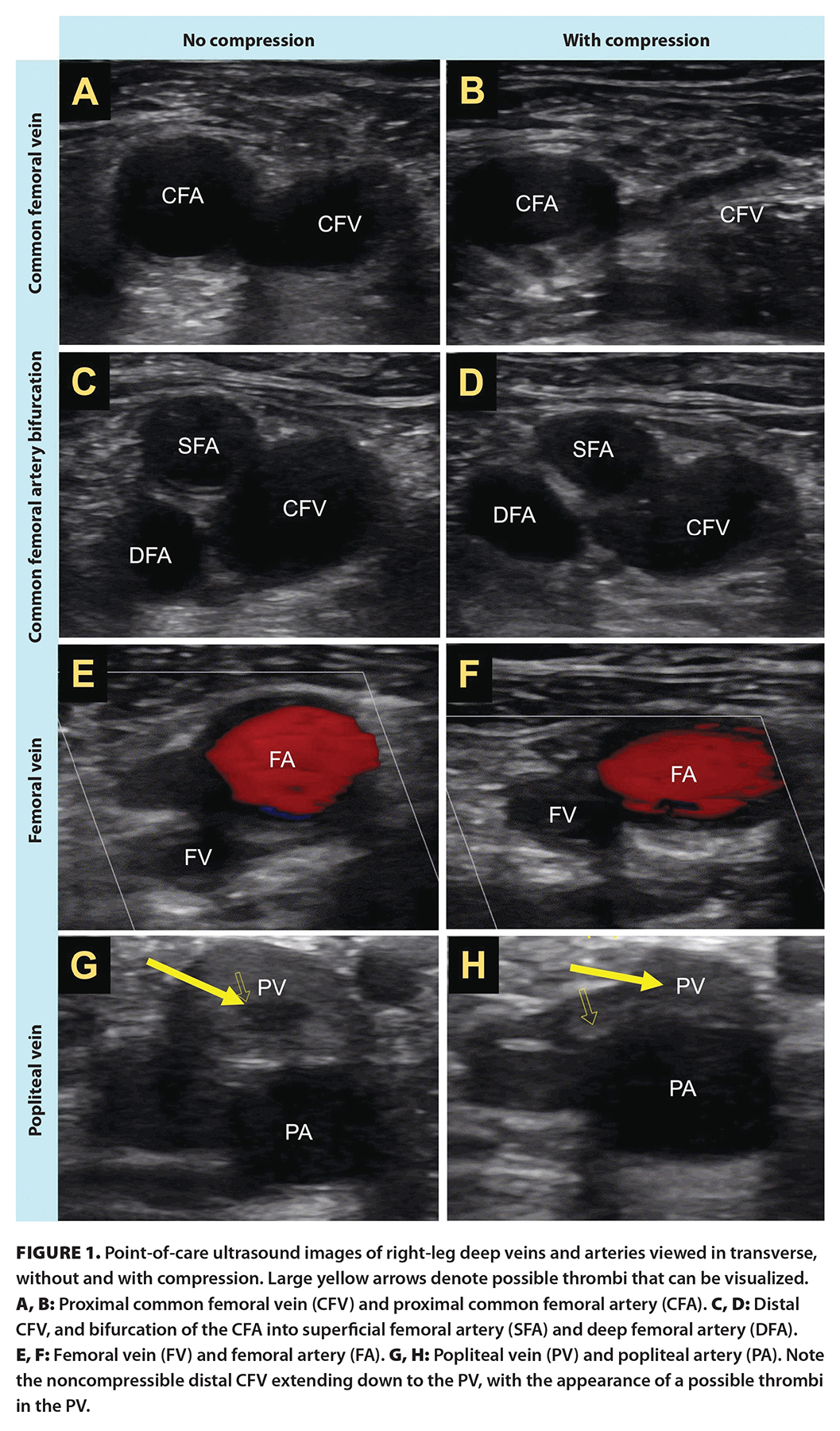 Lab results demonstrated an elevated D-dimer of 3.42 mcg/mL (normal < 0.31 mcg/mL) but no abnormalities in complete blood count, electrolytes, or creatinine/glomerular filtration rate. Troponin was negative; erythrocyte sedimentation rate was 10 mm/hour (normal is < 15 mm/hour), which was not indicative of the presence of inflammation; and venous blood gases showed pH 7.42, pCO2 45 mm Hg, and HCO3− 29 mmol/L. Electrocardiogram and chest X-ray showed no abnormalities. POCUS examination performed by the treating clinician revealed a right noncompressible common femoral vein with proximal extension from a partially occluding clot in the popliteal vein [Figure 1; Supplementary videos]. The POCUS cardiac exam was unremarkable and showed no signs of right heart strain.
Lab results demonstrated an elevated D-dimer of 3.42 mcg/mL (normal < 0.31 mcg/mL) but no abnormalities in complete blood count, electrolytes, or creatinine/glomerular filtration rate. Troponin was negative; erythrocyte sedimentation rate was 10 mm/hour (normal is < 15 mm/hour), which was not indicative of the presence of inflammation; and venous blood gases showed pH 7.42, pCO2 45 mm Hg, and HCO3− 29 mmol/L. Electrocardiogram and chest X-ray showed no abnormalities. POCUS examination performed by the treating clinician revealed a right noncompressible common femoral vein with proximal extension from a partially occluding clot in the popliteal vein [Figure 1; Supplementary videos]. The POCUS cardiac exam was unremarkable and showed no signs of right heart strain.
The top differential diagnoses for this patient’s dyspnea based on their history and clinical findings included pulmonary embolism and asthma exacerbation; the findings of deep vein thrombosis on POCUS examination further supported the likelihood of pulmonary embolism. CT and consultative ultrasound are not accessible in Haida Gwaii. Patients are required to take a ferry to Prince Rupert, which can result in delays ranging from 24 to 96 hours. Therefore, the patient was started on therapeutic anticoagulation with rivaroxaban 15 mg twice daily for a likely deep vein thrombosis and pulmonary embolism, as well as treatment for possible asthma exacerbation, which included regular inhaled salbutamol, fluticasone, and a 6-day course of prednisone 50 mg daily. He was referred for an urgent CT pulmonary angiogram and duplex lower-extremity ultrasonography, which was scheduled for approximately 1 week following his initial presentation.
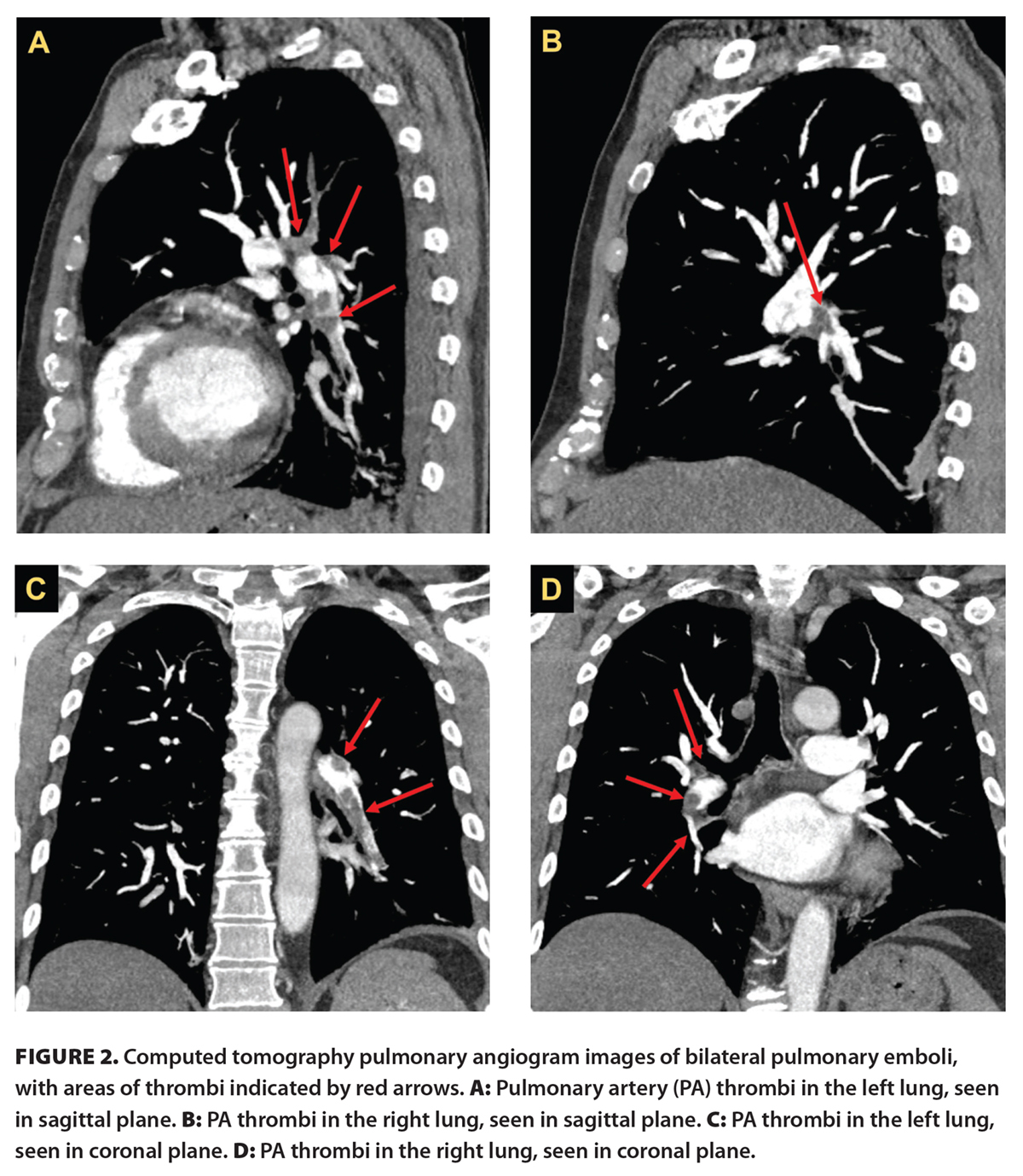 The patient reported improvement in his symptoms within days following initiation of anticoagulation and asthma exacerbation treatment. He had bilateral pulmonary emboli with proximal extension on CT pulmonary angiogram [Figure 2] and multiple thrombi in the right lower extremity on duplex ultrasonography, including from the popliteal vein to the common femoral vein, in the deep femoral vein, and in one branch of the duplicated superficial femoral vein. On follow-up 4 months after the initiation of treatment, the patient reported that all symptoms had resolved. He has continued anticoagulation following this initial encounter without experiencing any adverse events and will remain anticoagulated indefinitely. The patient has since undergone a hypercoagulability workup with his primary care provider, the results of which were inconclusive. Overall, the patient was satisfied with the diagnostic process and the prompt testing and management initiated.
The patient reported improvement in his symptoms within days following initiation of anticoagulation and asthma exacerbation treatment. He had bilateral pulmonary emboli with proximal extension on CT pulmonary angiogram [Figure 2] and multiple thrombi in the right lower extremity on duplex ultrasonography, including from the popliteal vein to the common femoral vein, in the deep femoral vein, and in one branch of the duplicated superficial femoral vein. On follow-up 4 months after the initiation of treatment, the patient reported that all symptoms had resolved. He has continued anticoagulation following this initial encounter without experiencing any adverse events and will remain anticoagulated indefinitely. The patient has since undergone a hypercoagulability workup with his primary care provider, the results of which were inconclusive. Overall, the patient was satisfied with the diagnostic process and the prompt testing and management initiated.
Discussion
The accurate and rapid diagnosis of venous thromboembolism can be challenging in rural settings and smaller communities due to the limited accessibility of consultative diagnostic imaging. A prompt diagnosis is required to initiate treatment for patients with venous thromboembolism, but there are several diagnostic challenges that may make prompt and accurate diagnosis difficult. Clinical findings alone have poor predictive accuracy because the development of symptoms of deep vein thrombosis depends on multiple factors, including the location of the thrombus, patency of collateral vessels, and degree of associated vascular inflammation.[2] The clinical presentation of pulmonary embolism also encompasses a wide spectrum, which can range from mildly symptomatic to sudden death.[14] To aid in the diagnosis of venous thromboembolism, using a validated pretest probability assessment model such as the Wells score may be helpful. The Wells score helps guide the decision about obtaining further investigations by estimating the likelihood of venous thromboembolism based on a set of defined criteria, which may reduce the burden of unnecessary investigations in patients with a low pretest probability.[15]
![TABLE. Wells criteria for pulmonary embolism. Low risk: score < 2 (1.3% prevalence); moderate risk: score 2–6 (16.2%); high risk: score > 6 (37.5%).[15]](https://bcmj.org/sites/default/files/BCMJ_Vol66_No8_venous-thromboembolism_web_Table.jpg) This case report showcases the utility of POCUS for diagnosing venous thromboembolism in a rural setting when consultative imaging is not readily accessible. It should be noted that based on the patient’s clinical presentation alone, there was a reasonable suspicion of pulmonary embolism, and anticoagulation would likely have been initiated without the POCUS findings. The patient’s Wells score was 6.0, which placed him in the moderate-risk category [Table], with points for a heart rate higher than 100 beats/minute, previously diagnosed deep vein thrombosis, and pulmonary embolism being as likely as the alternative diagnosis. However, the POCUS findings strongly improved the confidence of the diagnosis, which is crucial when deciding to initiate anticoagulation, given the potentially significant bleeding risks. A considerable benefit in the integration of POCUS in the clinical diagnosis of deep vein thrombosis is that positive findings improve diagnostic accuracy with a positive likelihood ratio of 30, making the diagnosis nearly certain.[9] The likelihood ratio of an adequately performed POCUS scan with negative findings is 0.04, which markedly lowers the probability of a deep vein thrombosis.[9] This suggests that POCUS findings have the potential to significantly influence posttest probabilities, and they carry considerable weight when used in clinical assessment for establishing or excluding the diagnosis of deep vein thrombosis.
This case report showcases the utility of POCUS for diagnosing venous thromboembolism in a rural setting when consultative imaging is not readily accessible. It should be noted that based on the patient’s clinical presentation alone, there was a reasonable suspicion of pulmonary embolism, and anticoagulation would likely have been initiated without the POCUS findings. The patient’s Wells score was 6.0, which placed him in the moderate-risk category [Table], with points for a heart rate higher than 100 beats/minute, previously diagnosed deep vein thrombosis, and pulmonary embolism being as likely as the alternative diagnosis. However, the POCUS findings strongly improved the confidence of the diagnosis, which is crucial when deciding to initiate anticoagulation, given the potentially significant bleeding risks. A considerable benefit in the integration of POCUS in the clinical diagnosis of deep vein thrombosis is that positive findings improve diagnostic accuracy with a positive likelihood ratio of 30, making the diagnosis nearly certain.[9] The likelihood ratio of an adequately performed POCUS scan with negative findings is 0.04, which markedly lowers the probability of a deep vein thrombosis.[9] This suggests that POCUS findings have the potential to significantly influence posttest probabilities, and they carry considerable weight when used in clinical assessment for establishing or excluding the diagnosis of deep vein thrombosis.
An additional benefit of POCUS highlighted in this case is its reliability in detecting deep vein thrombosis, with the preliminary bedside findings subsequently confirmed by duplex ultrasonography. POCUS has been used to diagnose deep vein thrombosis at the bedside with high sensitivity and specificity comparable to that of duplex ultrasonography, which has been demonstrated across various settings and disciplines. While duplex ultrasonography has sensitivity of 96% and specificity of 94% to 98% in diagnosing deep vein thrombosis,[8] multiple studies have shown that POCUS has similar sensitivity of 86% to 96% and specificity of 90% to 97%.[9-13] Another advantage of POCUS is that it can be performed rapidly at the bedside using portable ultrasonography devices, which are more readily available in rural settings than other forms of diagnostic imaging. A recent survey of rural BC practitioners showed that POCUS devices are easily accessed locally, with most respondents (87.5%) having access to an ultrasonography device.[16]
Common techniques for assessing deep vein thrombosis with POCUS are the two-region and three-region methods. The two-region method assesses the common femoral vein and popliteal vein; the three-region method includes an additional assessment of the femoral vein.[17,18] While the two-region method can be performed more rapidly than the three-region method, there is a risk of missing isolated deep vein thromboses within the femoral vein, which comprise 4% to 6% of all deep vein thromboses.[19-21] Therefore, the current recommendation is to use the three-region method over the two-region method to improve sensitivity when assessing deep vein thrombosis.
An important consideration in using POCUS is the potential for false-positive and false-negative results when assessing deep vein thrombosis. False-positive results may arise from signs of chronic deep vein thrombosis, such as scarring and fibrous tissue, which can be misinterpreted as acute deep vein thrombosis. False positives may also occur due to user error, including inadequate or off-axis compression.[22] The consequences of false-positive findings are substantial, because inappropriate anticoagulation can result in a burden of financial costs, inconvenience, medication interactions, and increased bleeding risk, including potentially life-threatening major bleeding. False-negative results are also consequential and can result from excessive probe pressure or failure to adequately assess the extent of the deep venous system.[22] A missed deep vein thrombosis increases the risk of developing a pulmonary embolism, which could be fatal if left untreated. To mitigate these limitations, POCUS should always be used in conjunction with other pretest probability assessments, including the Wells score and D-dimer. Obtaining further investigations only in patients with a high Wells score and elevated D-dimer can help reduce the risk of false-positive results.
Other limitations of POCUS include its lower sensitivity in diagnosing pulmonary embolism. In this case report, there were no findings of pulmonary embolism on lung or cardiac POCUS examinations; the diagnosis was suspected based on patient history, presenting symptoms, and venous imaging. The sensitivities of different ultrasounds in detecting deep vein thrombosis are 44% for venous ultrasound using the two-region compression method, 63% for cardiac ultrasound, and 81% for lung ultrasound.[23] Multi-organ POCUS, which combines venous, cardiac, and lung ultrasounds, yields a higher sensitivity of 92% in diagnosing pulmonary embolism;[23,24] therefore, it can be used in rural settings with other pretest probability assessments to more accurately diagnose pulmonary embolism.
The main challenge with implementing the use of POCUS is that it requires dedicated training as well as regular use and exposure for providers to maintain their skills. This is a relevant issue among rural practitioners in BC, who report that the most significant barriers to using POCUS include a lack of training and concerns about both funding and availability of training courses. In a recent survey, more than 40% of rural practitioners in BC reported receiving no diagnostic POCUS training in either undergraduate medical education or residency. There is a strong consensus among rural practitioners that increased funding and integration of formal training for medical students and residents would support POCUS use in rural settings.[16] In recent years, POCUS training has been slowly implemented in BC undergraduate medical and residency curricula, with the most apparent developments evident in emergency medicine training. We advocate that formal POCUS training be integrated into family medicine training as well, to support the diagnostic capabilities of future physicians working in rural communities and prevent misdiagnosis of venous thromboembolism.
Summary
POCUS is a practical and reliable method for diagnosing venous thromboembolism, particularly deep vein thrombosis. It can be used in settings where consultative diagnostic imaging is not readily available; when performed by trained personnel, it has high sensitivity and specificity comparable to that of duplex ultrasonography. In the rural setting in this case report, the use of POCUS aided in the diagnosis of deep vein thrombosis and pulmonary embolism, which resulted in the prompt and appropriate initiation of anticoagulation. However, there are notable funding and educational barriers to improving the use of POCUS in rural BC, which highlights the need for an integrated POCUS curriculum within medical undergraduate and residency training for family physicians.
Competing interests
None declared.
Supplementary videos
This article has been peer reviewed.
 |
| This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. |
References
1. Payne JG, Tagalakis V, Wu C, Lazo-Langner A. Current estimates of the incidence of acute venous thromboembolic disease in Canada: A meta-analysis. Thromb Res 2021;197:8-12.
2. Kearon C. Natural history of venous thromboembolism. Circulation 2003;107(23 Suppl 1):I22-I30.
3. Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: Clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999;353(9162):1386-1389.
4. Bĕlohlávek J, Dytrych V, Linhart A. Pulmonary embolism, part I: Epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp Clin Cardiol 2013;18:129-138.
5. Heit JA, Mohr DN, Silverstein MD, et al. Predictors of recurrence after deep vein thrombosis and pulmonary embolism: A population-based cohort study. Arch Intern Med 2000;160:761-768.
6. Schulman S, Lindmarker P, Holmström M, et al. Post-thrombotic syndrome, recurrence, and death 10 years after the first episode of venous thromboembolism treated with warfarin for 6 weeks or 6 months. J Thromb Haemost 2006;4:734-742.
7. Goodacre S, Sampson F, Thomas S, et al. Systematic review and meta-analysis of the diagnostic accuracy of ultrasonography for deep vein thrombosis. BMC Med Imaging 2005;5:6.
8. Bhatt M, Braun C, Patel P, et al. Diagnosis of deep vein thrombosis of the lower extremity: A systematic review and meta-analysis of test accuracy. Blood Adv 2020;4:1250-1264.
9. Pomero F, Dentali F, Borretta V, et al. Accuracy of emergency physician–performed ultrasonography in the diagnosis of deep-vein thrombosis: A systematic review and meta-analysis. Thromb Haemost 2013;109:137-145.
10. Mumoli N, Vitale J, Giorgi-Pierfranceschi M, et al. General practitioner-performed compression ultrasonography for diagnosis of deep vein thrombosis of the leg: A multicenter, prospective cohort study. Ann Fam Med 2017;15:535-539.
11. Canakci ME, Acar N, Bilgin M, Kuas C. Diagnostic value of point-of-care ultrasound in deep vein thrombosis in the emergency department. J Clin Ultrasound 2020;48:527-531.
12. Pedraza García J, Valle Alonso J, Ceballos García P, et al. Comparison of the accuracy of emergency department-performed point-of-care-ultrasound (POCUS) in the diagnosis of lower-extremity deep vein thrombosis. J Emerg Med 2018;54:656-664.
13. Kory PD, Pellecchia CM, Shiloh AL, et al. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest 2011;139:538-542.
14. Lavorini F, Di Bello V, De Rimini ML, et al. Diagnosis and treatment of pulmonary embolism: A multidisciplinary approach. Multidiscip Respir Med 2013;8:75.
15. Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: Management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med 2001;135:98-107.
16. Morton T, Kim DJ, Deleeuw T, et al. Use of point-of-care ultrasound in rural British Columbia: Scale, training, and barriers. Can Fam Physician 2024;70:109-116.
17. Theophanous RG, Chow VW, Convissar DL, et al. Point-of-care ultrasound screening for proximal lower extremity deep venous thrombosis. J Vis Exp 2023;(192):e64601.
18. Needleman L, Cronan JJ, Lilly MP, et al. Ultrasound for lower extremity deep venous thrombosis: Multidisciplinary recommendations from the Society of Radiologists in Ultrasound consensus conference. Circulation 2018;137:1505-1515.
19. Tabbut M, Ebersole N, Icken L, et al. Two-point compression ultrasound technique risks missing isolated femoral vein DVTs. West J Emerg Med 2022;23:597-600.
20. Rudnin S, Kaminsky J, Ghosh R, et al. Distribution of lower extremity deep vein thrombosis and implications for limited compression ultrasound examinations. J Emerg Med 2022;63:348-354.
21. Adhikari S, Zeger W, Thom C, Fields JM. Isolated deep venous thrombosis: Implications for 2-point compression ultrasonography of the lower extremity. Ann Emerg Med 2015;66:262-266.
22. Varrias D, Palaiodimos L, Balasubramanian P, et al. The use of point-of-care ultrasound (POCUS) in the diagnosis of deep vein thrombosis. J Clin Med 2021;10:3903.
23. Falster C, Jacobsen N, Coman KE, et al. Diagnostic accuracy of focused deep venous, lung, cardiac and multiorgan ultrasound in suspected pulmonary embolism: A systematic review and meta-analysis. Thorax 2022;77:679-689.
24. Daley JI, Dwyer KH, Grunwald Z, et al. Increased sensitivity of focused cardiac ultrasound for pulmonary embolism in emergency department patients with abnormal vital signs. Acad Emerg Med 2019;26:1211-1220.
Dr Zhuang was a fourth-year medical student at the University of British Columbia when this article was submitted. She is currently a first-year resident in internal medicine at the University of Alberta. Dr Morton is a clinical assistant professor in the Department of Family Practice at UBC and a staff physician at Haida Gwaii Hospital.
Corresponding author: Dr Rebecca Zhuang, rzhuang0@student.ubc.ca.