Health outcomes of patients in the Complex Chronic Diseases Program
ABSTRACT
Background: Complex chronic diseases affect almost 3% of Canadians and lead to persistent, debilitating symptoms. The BC Ministry of Health funded the Complex Chronic Diseases Program to address service gaps for affected individuals. We evaluated health outcomes of the program’s patients.
Methods: Analysis of data from the Complex Chronic Diseases Program Data Registry (June 2017–September 2022) focused on patient-reported outcomes and clinical measures at baseline, 6-month follow-up, and discharge, and on changes in symptoms across these time points.
Results: Among the 668 participants included in the study, slight improvements in overall physical and mental health were observed between baseline and discharge. However, symptoms such as sleep dysfunction, fatigue, and pain showed no significant changes.
Conclusions: While participation in the Complex Chronic Diseases Program yielded some health benefits, further research and interventions are required to address symptoms and optimize patient outcomes. The further development and use of objective outcome markers are needed for improved program evaluation.
New approaches and research are urgently needed to improve therapeutic interventions for patients with complex chronic diseases.
Background
Complex chronic diseases affect approximately 2.9% of Canadians, or approximately 855 000 people 12 years of age or older.[1] These diseases include myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), fibromyalgia, and chronic Lyme-like syndrome. ME/CFS manifests as persistent, disabling fatigue and general malaise (e.g., feeling unwell, flu-like symptoms), which are worsened by physical or mental exertion, and involves sleep dysfunction and impaired cognitive function that typically presents as “brain fog” or difficulty concentrating and processing information.[2] Fibromyalgia is characterized by widespread chronic pain that persists for at least 3 months, with patients frequently experiencing increased sensitivity to pain (hyperalgesia) or pain from typically nonpainful stimuli (allodynia).[3] Chronic Lyme-like syndrome develops following acute Lyme disease; it shares many symptoms with ME/CFS and other postviral conditions and with fibromyalgia.[4] Patients with complex chronic diseases often have persistent and debilitating symptoms coupled with functional impairment.[5,6] They experience great difficulty accessing consistent medical care in a coordinated fashion within the health care system, which frequently leaves them to advocate for themselves.
Complex Chronic Diseases Program
British Columbia’s Complex Chronic Diseases Program (CCDP), established in 2012 at BC Women’s Hospital and Health Centre in Vancouver, was the first tertiary care service of its kind in Canada and remains one of only three centres dedicated to the care of individuals with complex chronic diseases. The CCDP model of care includes diagnostic assessments, treatment planning, patient and caregiver education, and linkages to community and primary care resources. The program provides specialist-led care by a multidisciplinary team that includes physicians, a naturopath, and a pharmacist, who are supported by an interprofessional team of nurses, physiotherapists, occupational therapists, a dietitian, a social worker, and counseling services.
At inception, there were limited models to inform the CCDP, and care methods were continually revised to respond to the large volume of patients seeking care and the needs of the patient population. Initially, care was delivered primarily through one-on-one consultations with physicians and other clinicians. In 2019, the program moved to a group-based care model led by the interprofessional team, with individual medical visits provided based on patient needs, in response to increasing wait times and patient volume.[7] Due to continued high demand, the current wait time for care is approximately 28 months.
Symptom management: Referrals to the CCDP must be made by a clinician, typically a family physician, based on a suspected or confirmed diagnosis of ME/CFS, fibromyalgia, or chronic Lyme-like syndrome. Diagnosis at the CCDP is based on clinical criteria, including either the 2003 Canadian Consensus Criteria[2] or the 2015 Institute of Medicine Diagnostic Criteria[8] for diagnosis confirmation of ME/CFS and the 2016 Fibromyalgia Diagnostic Criteria for fibromyalgia.[3] For chronic Lyme-like syndrome, the diagnosis requires reliable laboratory evidence of Lyme infection in the past and symptoms akin to ME/CFS.
Remission rates are low for complex chronic diseases; few patients return to their premorbid functional or activity levels.[9] In the absence of curative therapies, current treatment guidance focuses on symptom management. The CCDP has treatment guidelines for clinical management informed by evidence, which are available on its website.
Patient education and self-management are central to the CCDP model of care. A variety of resources and services are offered to patients through group activities. Patients participate in group sessions that cover key aspects of managing symptoms, including fatigue, postexertional malaise, pain, sleep dysfunction, and impaired cognitive function. Patient education and self-management approaches have been employed in many contexts and are intended to help individuals learn and apply their knowledge and skills to managing their condition, mitigating its impact on daily life, and improving symptom severity and functional outcomes.
Complex Chronic Diseases Program Data Registry: Launched in 2017, the CCDP Data Registry supports program evaluation, quality improvement, and clinical research by tracking patient outcomes and demographics. It enables the assessment of symptom progression using clinical chart data and patient-reported questionnaires collected throughout the program and up to 6 months postcompletion.
The CCDP Data Registry provided a unique opportunity to characterize BC patients with complex chronic diseases and evaluate the longitudinal outcomes of service-based care. This study provides evidence of the benefits and limitations of current care strategies for patient outcomes and highlights areas that require more focused intervention, such as persistent symptoms of fatigue, pain, and sleep disturbances.
Methods
The University of British Columbia Research Ethics Board approved this study (H16-01648). Informed consent was obtained from all subjects involved in the study.
Data Registry participants
The CCDP Data Registry included newly referred individuals who were contacted by the CCDP, were 19 years of age or older, could read and understand English, and provided informed consent to be part of the data registry. Patients were invited to participate prior to their intake assessment with a CCDP physician. Exclusion criteria included not completing the standardized questionnaires prior to intake and ineligibility for the CCDP clinical program (e.g., lack of a qualifying diagnosis).
Following ethics approval, recruitment was conducted from June 2017 to September 2022 but was paused from January to June 2019 during a care model redesign and from March 2020 to February 2021 due to COVID-19 disruptions. The COVID pandemic prompted major changes to clinical processes at BC Women’s, including the adoption of telehealth and physical distancing requirements.
Data collection
Demographic information was collected prior to a patient’s first in-person appointment at the CCDP. Clinical variables were captured by the Interprofessional Assessment Tool, which was completed collaboratively by physicians and allied health professionals. The Interprofessional Assessment Tool contains data about complex chronic diseases diagnostic instruments, clinical variables, health history, symptom presentation, functional status, and physical examinations.
Standardized questionnaires were used to assess patient health outcomes four times: at program entry, after 6 months, at discharge (typically after 1 year), and 3 to 6 months postdischarge. The following questionnaires were used:
- Fatigue Severity Scale—measures the impact of fatigue on daily life.[10]
- Short-form McGill Pain Questionnaire—assesses the sensory and affective dimensions of pain.[11]
- Pittsburgh Sleep Quality Index (PSQI)—assesses nighttime sleep problems and sleep quality.[12]
- RAND 36-Item Short Form Health Survey (SF-36)—measures overall quality of life, summarized into mental health and physical health scores (higher scores indicate better well-being).[13]
- Patient Health Questionnaire 9 (PHQ-9) and Generalized Anxiety Disorder 7 (GAD-7)—assess the severity of depression and anxiety, respectively.[14,15]
- Patient Phenotyping Questionnaire Short Form (PQ-12)—captures the presence and severity of ME/CFS-specific symptoms (higher scores indicate worse severity).[16]
Inventory subscales and global scores were calculated based on the instrument’s cited calculation methods. Incomplete responses were excluded if key data were missing.
Data analysis
To ensure sufficient statistical power, some demographic variables were grouped into fewer categories. Descriptive statistics were used to characterize the patient population; means and standard deviations (or medians and interquartile ranges) were reported for continuous variables, and proportions were reported for categorical variables. Longitudinal symptom trends were visualized using sample means at each point in time.
Paired t tests were used to assess changes in symptoms between baseline and 6 months and between baseline and discharge. Tables were used to display sample sizes at each point in time to account for loss to follow-up. Normality was verified using quantile–quantile plots.
A series of univariable and multivariable linear regressions were used to examine whether disease duration at recruitment influenced baseline symptom severity and symptom changes from baseline to discharge. A confounding model approach was used, and confounders were included based on theoretical relevance or statistical criteria (i.e., association with both the outcome and the main exposure). In addition, the Akaike Information Criterion (AIC) was used to compare models, with the model showing the lowest AIC considered the best balance of goodness of fit and model complexity, helping to avoid overfitting and support robust model selection. Regression assumptions (linearity, normality, homoscedasticity, and independence) were verified.
To assess the effect of the 2019 transition from individual-based to group-based care, patients were categorized based on their assessment date (before or after 17 January 2019). Two-sample t tests were used to compare symptom changes from baseline to 6 months (discharge data were excluded due to a small sample size for the individual-based group: n = 35).
A two-sided P < .05 was considered statistically significant, and outcome means were reported with 95% confidence intervals. Analyses were conducted using R 3.2.3 software (R Foundation, Vienna, Austria).
The data presented in this study are available on request from the corresponding author.
Results
Recruitment
Participation in the CCDP Data Registry was offered to 1962 newly referred patients prior to confirmation of their eligibility for the CCDP; 735 consented to participate. The final analysis included 668 participants (34%) due to withdrawals and study exclusion criteria. The sample size was further reduced in some analyses due to missing data or loss to follow-up.
Baseline characteristics
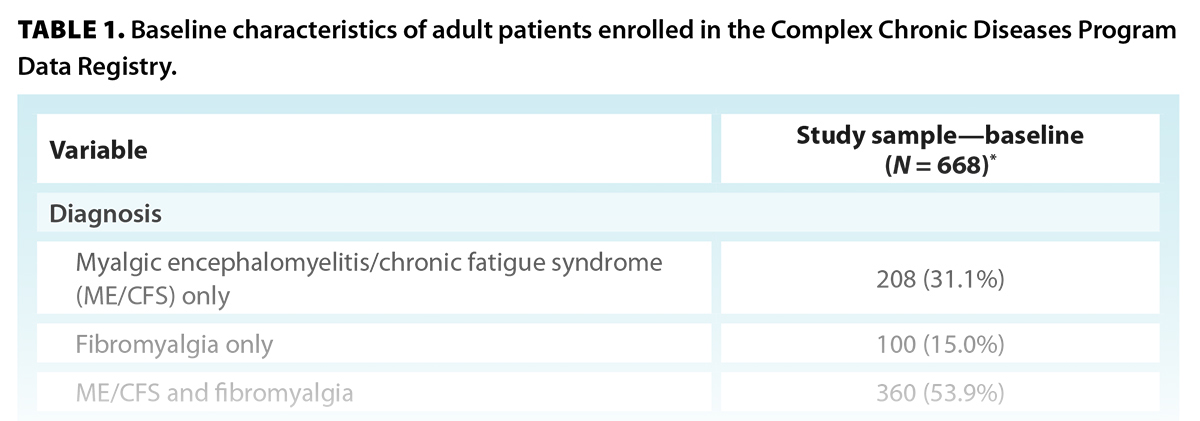
Diagnoses of ME/CFS and fibromyalgia were confirmed by the attending physician; 31% of participants were diagnosed with ME/CFS only (n = 208), 15% were diagnosed with fibromyalgia only (n = 100), and 54% were diagnosed with both ME/CFS and fibromyalgia (n = 360) [Table 1]. Only two participants had a confirmed diagnosis of chronic Lyme-like syndrome. One of them also had a diagnosis of ME/CFS and fibromyalgia and was categorized accordingly; the other was removed from the analyses.
Participants had an average age of 49 years. The sample was predominantly female (90%; n = 557) and predominantly self-identified as White/Caucasian (85%; n = 516) [Table 1].
Health outcomes
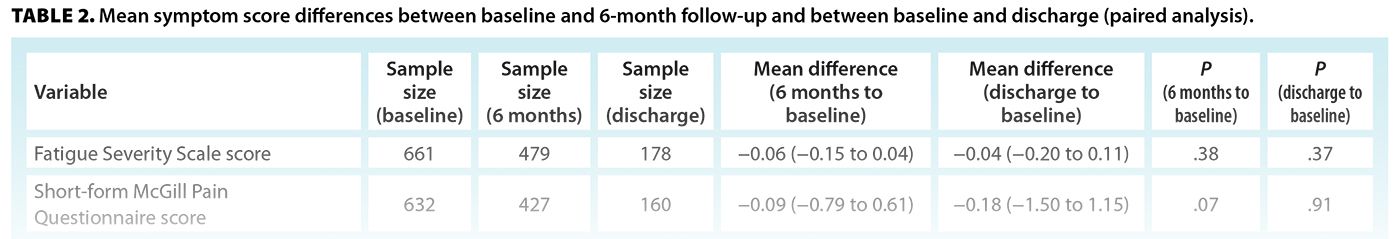
Measures of pain, fatigue, sleep dysfunction, and depression showed overall improvement but did not change significantly at 6-month follow-up or discharge [Table 2]. ME/CFS-specific symptoms, measured by the PQ-12, showed a significant reduction from baseline to discharge, suggesting overall symptom relief over time. Levels of anxiety (GAD-7) also decreased significantly at both 6-month follow-up and discharge. Both the SF-36 physical and mental health summary scores had improved significantly by discharge, indicating better physical and emotional well-being by the end of the program. Quantitative changes were small, and in the case of GAD-7, the confidence interval crossed zero, indicating a possible lack of clinical significance.
Disease duration
Longer disease duration was generally associated with worse baseline symptoms, with the McGill Pain Score and PSQI showing significant associations in univariable models [Table 3]. However, these associations weakened after adjusting for demographic factors: the PSQI remained the only significant predictor in the multivariable model. This suggests that each additional year of disease corresponded to a slight worsening of overall sleep quality (a PSQI score increase of 0.035 points).
The relationship between disease duration at baseline and symptom variable differences (discharge to baseline) were also examined, but no symptom variable changes retained a significant association after adjustment [Supplementary Table].
Model of care
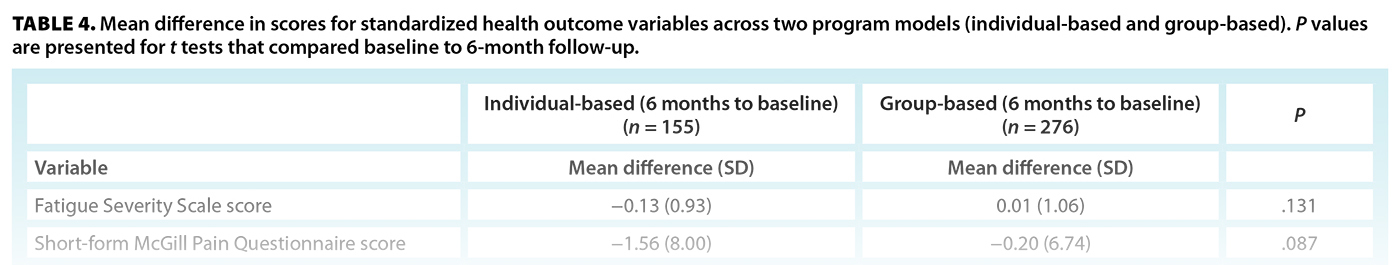
Based on their assessment date, patients were categorized as taking part in either the individual-based or group-based model of care. When the differences between baseline and 6-month scores were compared across the two groups, some mental health metrics were significantly different [Table 4]. Patients who took part in the individual-based model of care experienced a greater reduction in both anxiety (GAD-7) and depression (PHQ-9) severity than those in the group-based model, or an approximately 1.4-point decrease in scores from baseline to 6-month follow-up. For other symptoms, the individual-based model showed more favorable trends toward improvement; however, they were not significant.
Discussion
The CCDP Data Registry population aligns with other studies of complex chronic diseases. Approximately 88% of the sample identified as women, consistent with other research that reported more than 75% of ME/CFS and fibromyalgia patients as being women.[17-19] In the literature, fibromyalgia is commonly diagnosed during middle age (40 to 60 years of age),[20] while ME/CFS has two incidence peaks: in adolescence (10 to 19 years) and in adulthood (30 to 39 years).[21] However, the CCDP enrolls primarily long-standing cases and serves only adults (19+ years), which limits direct comparison to incidence rates.
Although complex chronic diseases affect all races and ages, most patients reported being White/Caucasian (85%), compared with 65.6% in the 2021 BC census.[22] The overrepresentation of White, middle-class individuals in clinical settings contrasts with population-based studies, which suggests that complex chronic diseases may be more common among people of lower socioeconomic status and minority cultural or ethnic groups.[23] These discrepancies may reflect differential health access and/or research participation rates and thus warrant further investigation.
Our results highlight the severity and persistence of illness and impairment faced by those with complex chronic diseases. Self-reported pain, fatigue, and mental health measures were substantially worse compared with a healthy population. For example, the Fatigue Severity Scale typically considers a score of 4 or higher (out of 7) as clinically significant fatigue; CCDP participants had an average score of 6, indicating that most participants were severely fatigued across all time points. Given the discrepancy between the well-being of individuals with complex chronic diseases and the well-being of both the general population and other chronic illness populations,[6,24] there is a clear need for interventions that are more finely tuned to the nuances of these conditions.
Our findings suggest a general trend in symptom improvement, including statistically significant changes in overall physical health and mental health indicators and severity of ME/CFS-specific symptoms between baseline and discharge. However, the magnitude of these changes was small, indicating that the improvements may not be clinically significant for most patients. The slight benefits to physical and emotional well-being may have been due to the educational and supportive aspects of the program and the tailored pharmaceutical approaches and resources for symptom management.
Our findings did not reveal a relationship between disease duration at intake and baseline symptom scores or symptom trajectory between baseline and discharge, except for PSQI scores, where longer disease duration was associated with poorer sleep quality. While symptom improvement is possible over time and with treatment, there are no curative treatments for complex chronic diseases, and prognosis for ME/CFS and fibromyalgia remains poor, particularly beyond the first year of illness.[25-27] Studies have been inconsistent in both methods and results, but generally, longer complex chronic disease duration correlates with poorer health outcomes.[28-30] We may not have seen the impacts of greater disease duration in our study because most participants had long-standing illness (median = 11 years), so early-stage improvements were not observed. Additionally, program enrollment may have excluded those with more favorable early-stage outcomes and those who were most severely affected, which would limit the interpretation of treatment timing effects.
In our analysis, only mental health outcomes differed significantly between the individual-based and group-based models of care. Patients in the individual-based model experienced greater reductions in anxiety and depression at 6 months than those in the group-based model, possibly due to the more personalized support provided. However, external factors, particularly the COVID pandemic, during which most group-based patients received care, may have affected the results. The burden of the pandemic may have resulted in some inflation of mental health scores.
The CCDP stays informed on the latest treatment and care strategies for people with complex chronic diseases. However, evidence of effective interventions that are robust and reproducible is limited. Studies on group-based self-management programs for ME/CFS and fibromyalgia show inconsistent outcomes and levels of effectiveness.[31-33] Our results show that the CCDP also experienced mixed and modest improvements in health outcomes. New approaches and research are urgently needed to improve therapeutic interventions for complex chronic diseases. The use of objective outcome measures could enhance the assessment of program effectiveness; candidates for clinical use include biomarkers (e.g., serum creatine kinase, morning cortisol), hand grip strength, and inflammatory markers.[27] Further validation of such markers is required.
Our study’s strengths included the large baseline sample and comprehensive data collection that encompassed a range of variables. The repeated measures design allowed individual and aggregate patient trajectories to be tracked throughout the program. Additionally, the cohort was well defined, with ME/CFS and fibromyalgia diagnoses clinically confirmed and recorded by trained practitioners, which minimized misclassification and missing data.
Study limitations
Because the CCDP is a provincial referral centre, our findings can be cautiously applied to other BC adults with complex chronic diseases. However, our sample was less diverse than the general BC population, and our sample size at discharge was relatively small due to high attrition and the ongoing data collection for participants currently enrolled in the CCDP Data Registry. Differential attrition bias may have influenced our findings, despite adjustment efforts. Self-reported symptom measures are subject to recall bias and may be less sensitive than objective markers. Additionally, the lack of a control group and external factors limited our ability to isolate program effects.
A major challenge for complex chronic diseases research is appropriate case ascertainment, given the absence of diagnostic biomarkers. While CCDP patients are identified using established diagnostic protocols, symptom presentation of complex chronic diseases is nonspecific, which makes it difficult to ensure a standard phenotype and to distinguish these diseases from others with similar presentations. The reliance on clinical diagnoses, in the absence of biomarkers, introduces an element of variability that future research must address. Further research should focus on identifying biomarkers and objective outcome measures to improve diagnosis and treatment evaluation, as well as improving our understanding of factors that predict better responses to care.
Conclusions
Our analysis of the CCDP Data Registry provided novel insights into symptom progression of complex chronic diseases under a clinical program and opportunities for continued exploration. While slight improvements were observed in mental health and physical health indicators and the severity of ME/CFS-specific symptoms, symptoms remained severe and persistent over time, regardless of care model, thus highlighting the long-term nature of complex chronic diseases. Individual-based care was more effective in reducing anxiety and depression, but overall symptom relief was limited.
Our findings highlight the challenges of complex chronic disease management and the need for retaining individualized care options and achieving earlier diagnosis and intervention, particularly at the primary care level. We recommend a coordinated investment and expansion of services that integrate primary care, community providers, and specialist expertise and are supported by continuing education and research. Although challenging in an environment of limited resources, improving early intervention and access to expert guidance could enhance patient satisfaction, reduce morbidity, and generate long-term economic savings.
Acknowledgments
Dr Nacul wishes to extend gratitude to the Pacific Public Health Foundation (formerly the BCCDC Foundation for Public Health) for its generous funding support of the CCDP Data Registry from 1 April 2018 to 31 March 2023, and for its sponsorship of the principal investigator’s protected research time through the Research Scholar Award from 1 April 2023 to 31 March 2024. In addition, Dr Nacul is grateful to the BC Women’s Health Foundation for supporting his research. All authors thank the CCDP and Women’s Health Research Institute staff who contributed their expertise to the various stages of development of the program, the facilitation of data collection, and the delivery of interventions for and with the patient community. The authors also extend gratitude to Ms Sabina Dobrer, senior statistician at the Women’s Health Research Institute, for her expert advice on statistical methods and review of the manuscript’s data analysis section, and to all those within and outside the program, including patients and knowledge users who contributed to the CCDP Data Registry and the strategic direction plan.
Competing interests
None declared.
This article has been peer reviewed.
 |
| This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. |
References
1. Rusu C, Gee ME, Lagacé C, Parlor M. Chronic fatigue syndrome and fibromyalgia in Canada: Prevalence and associations with six health status indicators. Health Promot Chronic Dis Prev Can 2015;35:3-11. https://doi.org/10.24095/hpcdp.35.1.02.
2. Carruthers BM, Jain AK, De Meirleir KL, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: Clinical working case definition, diagnostic and treatment protocols. J Chronic Fatigue Syndr 2003;11:7-115. https://doi.org/10.1300/J092v11n01_02.
3. Wolfe F, Clauw DJ, Fitzcharles M-A, et al. 2016 revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 2016;46:319-329. https://doi.org/10.1016/j.semarthrit.2016.08.012.
4. Bai NA, Richardson CS. Posttreatment Lyme disease syndrome and myalgic encephalomyelitis/chronic fatigue syndrome: A systematic review and comparison of pathogenesis. Chronic Dis Transl Med 2023;9:183-190. https://doi.org/10.1002/cdt3.74.
5. Lavergne MR, Cole DC, Kerr K, Marshall LM. Functional impairment in chronic fatigue syndrome, fibromyalgia, and multiple chemical sensitivity. Can Fam Physician 2010;56:e57-e65.
6. Nacul LC, Lacerda EM, Campion P, et al. The functional status and well being of people with myalgic encephalomyelitis/chronic fatigue syndrome and their carers. BMC Public Health 2011;11:402. https://doi.org/10.1186/1471-2458-11-402.
7. Meagher E, Magel T, Boulter T, et al. Complex Chronic Diseases Program: Program description & health outcomes assessment from a clinical data registry. MedRxiv 2024. Preprint. https://doi.org/10.1101/2024.05.25.24307912.
8. Institute of Medicine of the National Academies. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: Redefining an illness. Washington, DC: National Academies Press; 2015. https://doi.org/10.17226/19012.
9. Ghali A, Lacout C, Fortrat J-O, et al. Factors influencing the prognosis of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Diagnostics (Basel) 2022;12:2540. https://doi.org/10.3390/diagnostics12102540.
10. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989;46:1121-1123. https://doi.org/10.1001/archneur.1989.00520460115022.
11. Melzack R. The short-form McGill Pain Questionnaire. Pain 1987;30:191-197. https://doi.org/10.1016/0304-3959(87)91074-8.
12. Buysse DJ, Reynolds CF 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193-213. https://doi.org/10.1016/0165-1781(89)90047-4.
13. Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ 1993;2:217-227. https://doi.org/10.1002/hec.4730020305.
14. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-613. https://doi.org/10.1046/j.1525-1497.2001.016009606.x.
15. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med 2006;166:1092-1097. https://doi.org/10.1001/archinte.166.10.1092.
16. Lacerda EM, Mudie K, Kingdon CC, et al. The UK ME/CFS biobank: A disease-specific biobank for advancing clinical research into myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol 2018;9:1026. https://doi.org/10.3389/fneur.2018.01026.
17. Vaes AW, Van Herck M, Deng Q, et al. Symptom-based clusters in people with ME/CFS: An illustration of clinical variety in a cross-sectional cohort. J Transl Med 2023;21:112. https://doi.org/10.1186/s12967-023-03946-6.
18. Nacul LC, Lacerda EM, Pheby D, et al. Prevalence of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) in three regions of England: A repeated cross-sectional study in primary care. BMC Med 2011;9:91. https://doi.org/10.1186/1741-7015-9-91.
19. Wolfe F, Clauw DJ, Fitzcharles M-A, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600-610. https://doi.org/10.1002/acr.20140.
20. Häuser W, Ablin J, Fitzcharles M-A, et al. Fibromyalgia. Nat Rev Dis Primers 2015;1:15022. https://doi.org/10.1038/nrdp.2015.22.
21. Bakken IJ, Tveito K, Gunnes N, et al. Two age peaks in the incidence of chronic fatigue syndrome/myalgic encephalomyelitis: A population-based registry study from Norway 2008–2012. BMC Med 2014;12:167. https://doi.org/10.1186/s12916-014-0167-5.
22. Statistics Canada. Census profile, 2021 census of population. Updated 15 November 2023. Accessed 5 January 2024. www12.statcan.gc.ca/census-recensement/2021/dp-pd/prof/index.cfm?Lang=E.
23. Dinos S, Khoshaba B, Ashby D, et al. A systematic review of chronic fatigue, its syndromes and ethnicity: Prevalence, severity, co-morbidity and coping. Int J Epidemiol 2009;38:1554-1570. https://doi.org/10.1093/ije/dyp147.
24. Falk Hvidberg M, Brinth LS, Olesen AV, et al. The health-related quality of life for patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). PLoS One 2015;10:e0132421. https://doi.org/10.1371/journal.pone.0132421.
25. Walitt B, Fitzcharles M-A, Hassett AL, et al. The longitudinal outcome of fibromyalgia: A study of 1555 patients. J Rheumatol 2011;38:2238-2246. https://doi.org/10.3899/jrheum.110026.
26. O’Boyle S, Nacul L, Nacul FE, et al. A natural history of disease framework for improving the prevention, management, and research on post-viral fatigue syndrome and other forms of myalgic encephalomyelitis/chronic fatigue syndrome. Front Med (Lausanne) 2022;8:688159. https://doi.org/10.3389/fmed.2021.688159.
27. Nacul L, Authier FJ, Scheibenbogen C, et al. European Network on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (EUROMENE): Expert consensus on the diagnosis, service provision, and care of people with ME/CFS in Europe. Medicina (Kaunas) 2021;57:510. https://doi.org/10.3390/medicina57050510.
28. Kidd E, Brown A, McManimen S, et al. The relationship between age and illness duration in chronic fatigue syndrome. Diagnostics (Basel) 2016;6:16. https://doi.org/10.3390/DIAGNOSTICS6020016.
29. Nyland M, Naess H, Birkeland JS, Nyland H. Longitudinal follow-up of employment status in patients with chronic fatigue syndrome after mononucleosis. BMJ Open 2014;4:e005798. https://doi.org/10.1136/bmjopen-2014-005798.
30. Schaefer CP, Adams EH, Udall M, et al. Fibromyalgia outcomes over time: Results from a prospective observational study in the United States. Open Rheumatol J 2016;10:109-121. https://doi.org/10.2174/1874312901610010109.
31. Friedberg F, Adamowicz J, Caikauskaite I, et al. Efficacy of two delivery modes of behavioral self-management in severe chronic fatigue syndrome. Fatigue 2016;4:158–174. https://doi.org/10.1080/21641846.2016.1205876.
32. Pinxsterhuis I, Sandvik L, Strand EB, et al. Effectiveness of a group-based self-management program for people with chronic fatigue syndrome: A randomized controlled trial. Clin Rehabil 2017;31:93-103. https://doi.org/10.1177/0269215515621362.
33. Turcotte K, Oelke ND, Whitaker G, et al. Multi-disciplinary community-based group intervention for fibromyalgia: A pilot randomized controlled trial. Rheumatol Int 2023;43:2201–2210. https://doi.org/10.1007/s00296-023-05403-5.
Ms Meagher and Ms Magel were research assistants at the Women’s Health Research Institute, BC Women’s Hospital and Health Centre, at the time of writing. Mr Boulter is a research coordinator at the Women’s Health Research Institute. Ms Muñoz is a research manager at the Women’s Health Research Institute. Mrs Prestley is a research and knowledge translation manager at the Women’s Health Research Institute. Dr Chan is an investigator at BC Children’s Hospital; head of the Department of Medicine, BC Women’s obstetric medicine lead, BC Women’s; and a clinical professor in the Department of Medicine, University of British Columbia. Ms Bryden was an occupational therapist in the Complex Chronic Diseases Program at BC Women’s at the time of writing. Dr Nacul is a clinical scientist at the Women’s Health Research Institute; a clinical associate professor in the Department of Family Practice, Faculty of Medicine, UBC; and a clinical associate professor in the Department of Clinical Research, CureME, London School of Hygiene & Tropical Medicine.
Corresponding author: Ms Emily Meagher, emily.meagher@cw.bc.ca.