Diagnosis and treatment of ectopic pregnancy
ABSTRACT: Ectopic pregnancy refers to implantation of an embryo outside the endometrium. It is a medical emergency, but associated maternal mortality has significantly declined over the decades due to earlier diagnosis and treatment. Timely detection of ectopic pregnancy is contingent on having a high index of suspicion in all women of reproductive age, identifying patient risk factors, and then performing appropriate laboratory testing and imaging. Expectant management is less commonly used than medical management, which is preferred for asymptomatic, vitally stable women who wish to avoid surgery. Minimally invasive surgery is the gold standard for management of unstable or ruptured ectopic pregnancy. Recent data favor salpingectomy for women with a healthy contralateral tube because it has higher treatment success and does not appear to reduce future fertility compared to salpingotomy. However, salpingotomy is suggested for women with a dysfunctional or absent contralateral tube, or those who elect to preserve both tubes and accept the increased risk of treatment failure. Knowledge of the risks and benefits of each treatment option is critical for delivering patient-centred care.
Early diagnosis of ectopic pregnancy is critical to reducing maternal mortality and improving treatment success rates, especially since many women have no identifiable risk factors.
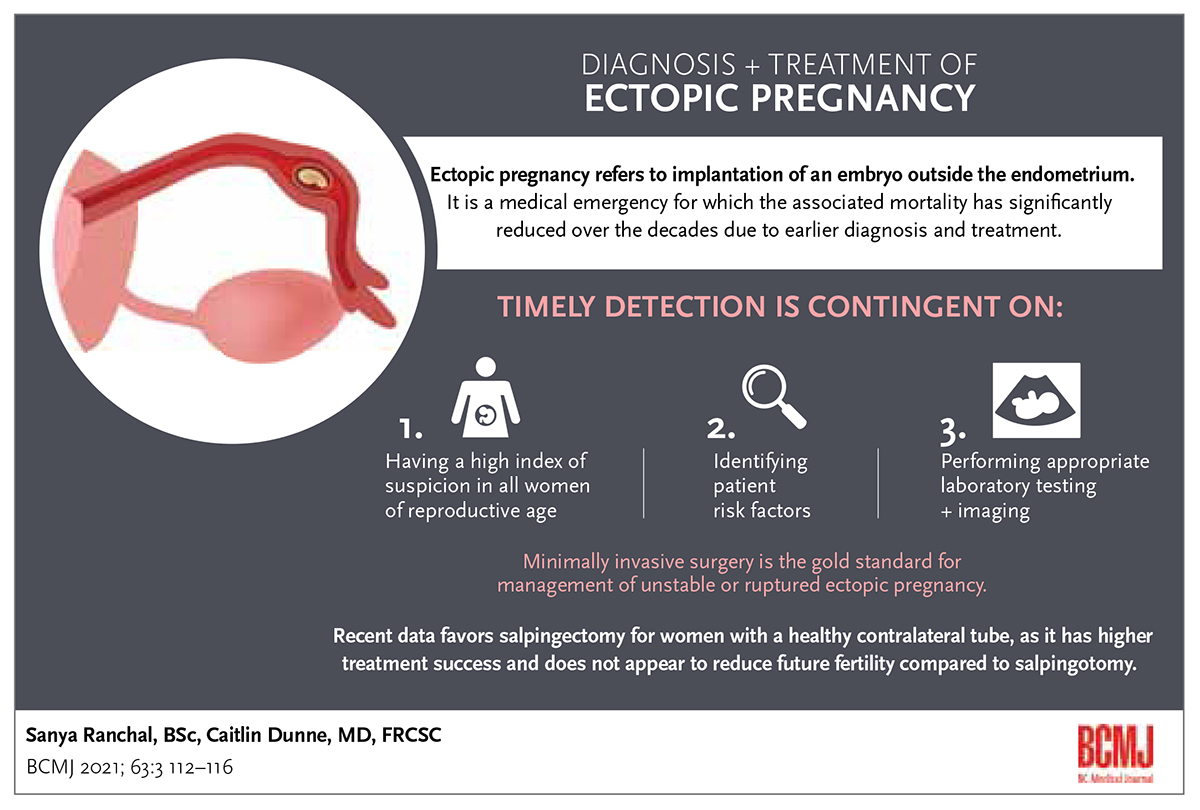
Ectopic pregnancy occurs when a developing embryo implants at a site other than the endometrium of the uterine cavity, most commonly within the fallopian tube. Although the incidence of ectopic pregnancy is estimated to be approximately 2% of all pregnancies, it is one of the most common gynecologic emergencies encountered by community physicians.[1] Ruptured ectopic pregnancy can lead to severe hemorrhage and is a significant cause of pregnancy-related maternal mortality in the first trimester.[2] Thus, timely diagnosis of ectopic pregnancy is essential to prevent maternal mortality and improve treatment outcomes.
Maternal mortality related to ectopic pregnancy has plummeted over the last two decades due to the availability of quantitative beta-human chorionic gonadotropin (b-hCG) testing, transvaginal ultrasound, and laparoscopy, which allow for early diagnosis and intervention.[1] Despite this, ectopic pregnancy and its treatments remain a prevalent cause of morbidity among women and can affect long-term reproductive success. With a comprehensive understanding of ectopic pregnancy, community physicians can help women make informed decisions and thus provide personalized health care. This review outlines the current practices, recent advances, and unresolved topics related to diagnosis, management, and prognosis of ectopic pregnancy.
Risk factors
Only half of the women who are diagnosed with ectopic pregnancy have identifiable risk factors.[2] Thus, it is critical to maintain a high index of suspicion in all women of reproductive age who present with amenorrhea, abdominal pain, irregular vaginal bleeding, or a history of ectopic pregnancy.[3] The pretest probability of ectopic pregnancy is increased if multiple risk factors are elicited when taking a history, which can aid in making a prompt diagnosis.
The most well-documented risk factor for an ectopic pregnancy is a previous ectopic pregnancy.[4,5] Women with a prior ectopic pregnancy have a 10-times higher risk of recurrence than the general population.[6] After one ectopic pregnancy, there is a 10% to 15% chance of recurrence, which increases to 25% in women who have had two or more ectopic pregnancies.[6] Recurrence can be attributed to congenital tubal dysfunction, acquired tubal damage from pelvic inflammatory disease, or previous tubal surgery—all of which may impede embryonic passage through the fallopian tube.[4,7] Women with perihepatic adhesions (commonly known as Fitz-Hugh-Curtis syndrome), a complication of pelvic inflammatory disease, carry twice the risk of ectopic pregnancy recurrence compared to unaffected women.[7]
Smoking, even “light” consumption of one to nine cigarettes per day, increases the risk of ectopic pregnancy by up to twofold.[8] Some other well-recognized risk factors for ectopic pregnancy are age over 35 years, history of infertility, prior tubal surgery, and laboratory/laparoscopy confirmed pelvic inflammatory disease.[2] Additionally, genital surgery, endometriosis, and dysmenorrhea have been recognized as significant risk factors.[4] Jacob and colleagues also described a 1.8-fold (95% CI 1.54-2.09) increase in the risk of ectopic pregnancy in women with a diagnosis of mental health disorders, including depression, anxiety, adjustment disorder, and somatoform disorder.[4] This finding might be limited to an association, confounded by increased rates of psychiatric disorders in women with a history of infertility, chronic pelvic pain, endometriosis, recurrent miscarriages, and so on. It is also possible that the medications used for treating such disorders disrupt embryo transport through the fallopian tube.[4] More studies are needed to understand the association between mental health and ectopic pregnancy before drawing definitive conclusions.
Older data associated intrauterine contraceptive devices (IUDs) with ectopic pregnancy.[9] And while it remains true that if pregnancy occurs with an IUD in situ the risk of ectopic pregnancy is high, all forms of contraception reduce the risk of pregnancy and ectopic pregnancy.[6] In vitro fertilization (IVF) was previously thought to be associated with increased risk of ectopic pregnancy due to possible underlying fallopian tube dysfunction in the infertile population and procedure-related factors.[2] The latter may not be relevant today because the IVF practices associated with increased rates of ectopic pregnancy, such as transfer of multiple embryos and day-3 embryo transfer, are less common in modern clinical practice. As a result, the incidence of ectopic pregnancy after IVF has decreased significantly, and many physicians now suggest that IVF pregnancies may be at little or no increased risk of ectopic pregnancy compared to natural conceptions.[6]
Diagnosis
History and presentation
Ectopic pregnancies almost always occur in the fallopian tube (> 95%), particularly in the ampulla (distal portion) (70%).[7] Fewer tubal pregnancies occur in the isthmus (middle portion) (12%) and fimbria (11%).[10] Rarely, pregnancies may grow in the cervix (< 1%) or abdomen (1%), or on the ovary (3%).[10,11] It is important to obtain a full history, including menstrual and obstetrical history, to determine gestational age and evaluate for risk factors in all women of reproductive age. Women with an ectopic pregnancy most commonly present with abdominal pain, vaginal bleeding, or both.[2] However, these are also symptoms of miscarriage, which is, by far, the most common cause of failing pregnancy and/or abnormally rising b-hCG levels. An ectopic pregnancy may be intact or ruptured at presentation; the latter might present with hemodynamic instability and an acute abdomen that requires urgent surgical management to address ongoing hemorrhage.[10] Initial workup includes confirmation of pregnancy (through urine or serum b-hCG testing) and a transvaginal ultrasound to determine the location of the pregnancy.[2]
Laboratory investigations
Serial quantitative serum b-hCG testing can be helpful in determining if the current pregnancy is likely to be in an ectopic location. In a normal pregnancy, the b-hCG level rises steeply for the first 4 weeks, followed by a slower rise until 10 weeks gestational age, with an eventual plateauing.[12] In most normal intrauterine pregnancies, the b-hCG level will rise 65% to 100% every 48 hours, although even a short plateau in b-hCG can be normal in rare cases.[2] When performing serial b-hCG measurements, it is recommended that the same laboratory be used to minimize the risk of interassay variability, which can be 5% to 10%.[10]
Decreasing b-hCG levels strongly suggest a failing pregnancy, but they do not indicate its location. If no intrauterine pregnancy has been confirmed, the woman should be closely monitored because it is possible for an ectopic pregnancy to rupture, even with very low b-hCG levels. The use of discriminatory b-hCG levels to determine when an intrauterine pregnancy should be visible on ultrasound is discouraged. Evidence from the 1980s suggested that b-hCG of 1000 to 2000 IU/L without a visible pregnancy could be assumed to be ectopic.[13] It is now widely acknowledged that b-hCG can be nonspecific, as many ectopic pregnancies will never reach a level of 2000 IU/L or might rupture before that threshold. Conversely, women who have had multiple gestations have higher b-hCG levels than women who have had a single gestation, and using 2000 IU/L as a discriminatory value might not be accurate for such pregnancies.[2] Historical use of a “threshold” has resulted in the treatment of intrauterine pregnancies with methotrexate, a chemotherapeutic agent; hence, newer studies have urged caution and patience when evaluating early pregnancies of uncertain viability.[13]
Imaging
Transvaginal ultrasound is the optimal method for imaging pregnancies in the early first trimester. In a normal pregnancy, a gestational sac is visualized at 5 weeks gestation (3 weeks after conception), when it is 2 to 5 mm in diameter.[13] Following that, the yolk sac is the earliest structure to develop inside the gestational sac and is normally seen by 5 weeks and 5 days of pregnancy.[10] Presence of an intrauterine pregnancy (gestational sac plus a yolk sac or embryo) on transvaginal ultrasound effectively eliminates the diagnosis of an ectopic pregnancy other than the rare scenario of a heterotopic pregnancy (one embryo within the uterus and another extra-uterine).[10] However, even with modern high-resolution ultrasound, it is rare that ultrasound alone is sufficient to be definitive. Without a yolk sac, an intrauterine pregnancy cannot be confirmed, and clinicians should be wary because it might represent a pseudosac—a fluid collection in the endometrial cavity caused by sloughing of the decidua.[10] To differentiate between a pseudosac and an early gestational sac, a follow-up ultrasound in 7 to 14 days should be arranged.
Transvaginal ultrasound can definitively diagnose an ectopic pregnancy if an extra-uterine gestational sac with yolk sac/embryo is visible[2] [Figure]. However, most ectopic pregnancies lack these definitive features on imaging and are often described as an inhomogeneous adnexal mass separate from the ovaries.[10] An adnexal mass might also represent a cyst, corpus luteum, or bowel.[2] The presence of hemoperitoneum (echogenic intraperitoneal fluid) and placental blood flow within the periphery of this mass (“ring of fire”) on color doppler can aid in diagnosis.[10]
Expectant management
Expectant management of ectopic pregnancy involves allowing the pregnancy to take its natural course with close physician follow-up until there is clinical resolution of symptoms, a negative urine pregnancy test, or negative serum b-hCG.[14] There is evidence that expectant management of ectopic pregnancy can be a safe option in a select population of women who are hemodynamically stable, asymptomatic, have a b-hCG value less than 1000 IU/L, with decreasing levels, and can reliably access regular physician follow-up.[15] These women can avoid the use of methotrexate and its possible side effects. It is worth noting, however, that a 5-year follow-up cohort study of 217 women who underwent expectant, medical, or surgical management of a first ectopic pregnancy suggested there was a 2.68 times higher risk of recurrent ectopic pregnancy in women who were managed expectantly.[5] A randomized study called ACTorNOT (ClinicalTrials.gov NCT02152696) has completed recruitment to compare expectant management versus uterine evacuation plus methotrexate versus methotrexate alone in women with ectopic pregnancy.
Medical treatment
Methotrexate, the most common option for treating ectopic pregnancy, was first used for this purpose in 1982.[16] It is a folate antagonist that prevents DNA replication and affects rapidly proliferating cells like that of a developing embryo.[17] A single dose of methotrexate is administered intramuscularly based on body surface area (50 mg/m2). Its effectiveness is assessed by serial b-hCG measurements on days 4 and 7 post-treatment, then weekly until resolution.[2] A reduction of less than 15% in b-hCG level between days 4 and 7 posttreatment may indicate that treatment is inadequate; therefore, a second dose of methotrexate might be required.[2] Close observation is required to ensure patient stability, declining b-hCG levels, and normal liver function tests because methotrexate can affect liver function.[2,17]
The b-hCG level at presentation is strongly associated with treatment success of a single dose methotrexate injection. A systematic review that analyzed five observational studies determined that women with a baseline b-hCG level of more than 5000 IU/L were 4 times more likely to have treatment failure with single-dose methotrexate than those with a presenting baseline between 2000 and 4999 IU/L.[18] Thus, most guidelines suggest using methotrexate to treat ectopic pregnancy in women with a presenting b-hCG level less than 5000 IU/L. Other factors such as ectopic mass > 3.5 cm and presence of fetal heartbeat on transvaginal ultrasound are considered relative contraindications to the use of medical therapy because they might indicate a more developed embryo, which implies increased risk of ectopic rupture.[2,10] However, few data are available to support these recommendations.[10]
One in three women may experience mild, self-limited side effects of methotrexate, including nausea, diarrhea, stomatitis, and conjunctivitis.[2] Serious complications, including anaphylaxis, pulmonary damage, and myelosuppression, have also been reported.[10] Since methotrexate can cause temporary hepatic dysfunction, it is important to obtain a CBC and baseline liver and renal function laboratory results, and to monitor liver function if indicated. Patients should also be advised to stop their folate-containing supplements because they inhibit methotrexate function.[10] Nonsteroidal anti-inflammatory medications should also be avoided because they may reduce renal clearance of the drug by reducing renal blood flow.[10] Alcohol should be avoided during methotrexate treatment to prevent the combined effect of hepatotoxic drugs. Methotrexate should not be administered to patients with liver or renal dysfunction, lung disease, hematologic dysfunction, immunodeficiency, or peptic ulcer disease, or to those who are breastfeeding. Given that this is an outpatient treatment, and an ectopic pregnancy may rupture during therapy, it is important to alert patients to the symptoms of a ruptured ectopic pregnancy and to seek immediate medical attention if they occur.
Advances in medical treatment of ectopic pregnancy may be on the horizon. Researchers from the University of British Columbia have demonstrated that gonadotropin-releasing hormone (GnRH) and its receptor are expressed in trophoblast cells and fallopian tube epithelium at ectopic pregnancy implantation sites.[19] This presents the potential to use a targeted and less toxic agent for conservative treatment of ectopic pregnancies. A randomized controlled trial comparing GnRH agonist versus methotrexate was registered in March 2020.[20] The trial is also planning to investigate the use of letrozole, an aromatase inhibitor that blocks the final step in estrogen synthesis, versus methotrexate to treat ectopic pregnancy, and is stated to conclude in 2022.
Surgical management
With improved laparoscopic instruments and techniques, minimally invasive surgery has become the gold standard for treating ectopic pregnancy and has mostly replaced laparotomy. Laparoscopic surgery offers a safer, faster, cheaper, and more esthetic option.[10,21] With improved operator experience, even stable but symptomatic ectopic pregnancies can be managed with laparoscopy, which can result in quicker hemostasis and better patient outcomes.[21] However, laparotomy is sometimes used for hemodynamically unstable cases because it might offer better field visualization when managing a large bleed.[10]
Two laparoscopic techniques are available for treating tubal pregnancies: salpingectomy, where the fallopian tube containing the ectopic pregnancy is removed, and salpingotomy, where after removal of the ectopic mass, the affected fallopian tube is preserved. There is ongoing debate about treatment success, future fertility, and risk of repeat ectopic pregnancy after treatment with salpingotomy versus salpingectomy.
Salpingectomy versus salpingotomy
Treatment success and future fertility
Given that salpingotomy requires the surgeon to meticulously extract a small trophoblastic mass while preserving the fallopian tube, the method might be prone to trophoblastic tissue retention, which can necessitate a salpingectomy. Multiple retrospective studies report trophoblast persistence rates between 9.0% and 12.0% for salpingotomy and 1.8% for salpingectomy.[22] In an open-label, randomized control trial named European Surgery in Ectopic Pregnancy, women with ultrasound-confirmed ectopic pregnancy who were eligible for surgical management were randomly assigned to either salpingotomy or salpingectomy. The trial reported significantly higher postsurgical trophoblast persistence in the salpingotomy group (n = 215) than in the salpingectomy group (n = 231) (RR 15.0, p = 0.01).[23] The trial also found no significant difference in rates of naturally conceived pregnancies 36 months postsurgery (fecundity ratio 1.06, p = 0.687).[23] Thus, for women with tubal pregnancy and a healthy contralateral tube, salpingectomy is a reasonable treatment option because it minimizes risk of ectopic mass persistence and does not seem to reduce future fertility. However, for women with contralateral tubal pathology or no contralateral tube, conservative treatment with salpingotomy should be considered if they wish to maintain the potential for natural conception.
Risk of recurrent ectopic pregnancy
Multiple studies have evaluated the risk of ectopic recurrence following salpingotomy versus salpingectomy, but no consensus has been reached. A 12-year retrospective study found a recurrent ectopic pregnancy rate of 13% in the ipsilateral tube following salpingotomy, while in the salpingectomy group, there were no recorded recurrences.[22] These data might be confounded by the fact that women who choose to undergo salpingotomy are more likely intending to conceive and have higher pregnancy rates compared to those who choose to undergo salpingectomy. Multiple retrospective studies that have included only women who are actively wanting to conceive post-salpingotomy or post-salpingectomy have reported no difference in rates of ectopic pregnancy recurrence between the two groups.[24,25] Thus, data on the rates of ectopic pregnancy recurrence after different surgical procedures are still conflicting.
Discussion
Medical versus surgical management
Patients who are asymptomatic and hemodynamically stable can be managed with either intramuscular methotrexate or laparoscopic surgery. The decision should be guided by patient characteristics, laboratory and radiological findings, and patient preference after discussion of the risks and benefits. When a patient has any contraindications to methotrexate use, surgical management is often necessary. Surgical management of a stable, asymptomatic patient might also be prudent if the patient wishes to concurrently undergo tubal sterilization or requests removal of a tube with recurrent ectopics.
It is important to clarify that no long-term effects on future fertility have been identified after methotrexate use or surgical treatment. It is common to suggest a 3-month waiting period post-methotrexate treatment before attempting to conceive again. This time frame appears to be somewhat arbitrary because studies have suggested that conception before the 3-month mark is no more likely to result in birth defects.[26] Among women intending to conceive, no significant difference in spontaneous intrauterine pregnancy rates have been found when comparing women previously treated with single dose methotrexate versus those who underwent surgical treatment for ectopic pregnancy.[17] One study reviewed 594 patients who achieved pregnancy using IVF after one or more ectopic pregnancies. Comparing women who were managed with unilateral salpingectomy to those managed with methotrexate indicated that the rates of ectopic pregnancy were equivalent (3.6% versus 2.8%; adjusted OR 1.4, 95% CI 0.5-3.8).[27] The rate of recurrence was most strongly associated with the number of previous ectopic pregnancies rather than the treatment modality used during those pregnancies.[27] Thus, risk of recurrence of ectopic pregnancies should not play a major role in decisions about treatment when comparing medical versus surgical options in eligible women.
Summary
Clinicians should be aware of the possibility of ectopic pregnancy for all women of reproductive age because early diagnosis is critical to reducing maternal mortality and improving treatment success rates. An understanding of the treatments, eligibility criteria, necessary follow-up, and pros and cons of each treatment option can help clinicians ensure patient safety and autonomy. Medical or expectant management is a safe and effective option for a carefully selected population of stable, asymptomatic women. Laparoscopy is the gold standard for surgical management of ectopic pregnancy, with salpingectomy having higher success rates than salpingotomy and comparable future fertility rates. Clinical presentation, ectopic size, b-hCG level, and patient preference are all important to consider when recommending treatment options for ectopic pregnancy because these factors may influence treatment success, risk of recurrent ectopic pregnancy, and short-term fertility.
Acknowledgments
The authors would like to acknowledge the College of Physicians and Surgeons’ librarians and library technicians for their assistance in conducting the literature search, which was critical to the production of this article.
Competing interests
Dr Dunne is a member of the BCMJ Editorial Board but did not participate in the review or decision making regarding this article. No competing interests declared.
Ectopic pregnancy—clinical pearls
- In most normal pregnancies, the b-hCG level rises 65% to 100% every 48 hours for the first 4 weeks.
- The yolk sac is the earliest structure to develop inside the gestational sac and is normally seen by 5 weeks and 5 days of pregnancy.
- An intrauterine gestational sac without a yolk sac or embryo is not sufficient to rule out ectopic pregnancy and might represent a pseudosac.
- Hemodynamically stable, asymptomatic women with a decreasing presenting b-hCG level < 1000 IU/L might be eligible for expectant management of ectopic pregnancy.
- Methotrexate is administered intramuscularly at a dose of 50 mg/m2 of body surface area.
- A reduction of < 15% in the b-hCG level between days 4 and 7 post-methotrexate may indicate that treatment is inadequate, and a second dose of methotrexate might be required.
This article has been peer reviewed.
References
1. Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol 2011;117:837-843.
2. Barnhart KT, Franasiak J. ACOG Practice Bulletin No. 193: Tubal ectopic pregnancy. Obstet Gynecol 2018;131:e91-e103.
3. Ramakrishnan K, Scheid DC. Ectopic pregnancy: Forget the “classical presentation” if you want to catch it sooner. J Fam Pract 2006;55:388-395.
4. Jacob L, Kalder M, Kostev K. Risk factors for ectopic pregnancy in Germany: A retrospective study of 100,197 patients. Ger Med Sci 2017;15:Doc19.
5. Ellaithy M, Asiri M, Rateb A, et al. Prediction of recurrent ectopic pregnancy: A five-year follow-up cohort study. Eur J Obstet Gynecol Reprod Biol 2018;225:70-78.
6. Ectopic pregnancy. In: Taylor HS, Pal L, Seli E. Speroff’s clinical gynecologic endocrinology and infertility. 9th ed. Philadelphia, PA: Wolters Kluwer; 2020. p.1260-1288.
7. Mullins E, Agarwal N, Oliver R, Odejinmi JF. Implications of perihepatic adhesions in women undergoing laparoscopic surgery for ectopic pregnancy. Int J Gynecol Obstet 2015;130:247-249.
8. Saraiya M, Berg CJ, Kendrick JS, et al. Cigarette smoking as a risk factor for ectopic pregnancy. Am J Obstet Gynecol 1998;178:493-498.
9. Franks AL, Beral V, Cates Jr W, Hogue CJ. Contraception and ectopic pregnancy risk. Am J Obstet Gynecol 1990;163(4 Pt 1):1120-1123.
10. Ectopic pregnancy. In: Hoffman BL, Schorge JO, Bradshaw KD, et al., editors. Williams gynecology. 3rd ed. New York: McGraw-Hill; 2016.
11. Dunne C, Havelock JC. Ovarian ectopic pregnancy after in vitro fertilization. J Obstet Gynaecol Can 2012;34:409.
12. Morin L, Cargill YM, Glanc P. Ultrasound evaluation of first trimester complications of pregnancy. J Obstet Gynaecol Can 2016;38:982-988.
13. Doubilet PM, Benson CB. Further evidence against the reliability of the human chorionic gonadotropin discriminatory level. J Ultrasound Med 2011;30:1637-1642.
14. Jurkovic D, Memtsa M, Sawyer E, et al. Single-dose systemic methotrexate vs expectant management for treatment of tubal ectopic pregnancy: A placebo–controlled randomized trial. Ultrasound Obstet Gynecol 2017;49:171-176.
15. Odejinmi F, Huff KO, Oliver R. Individualisation of intervention for tubal ectopic pregnancy: Historical perspectives and the modern evidence based management of ectopic pregnancy. Eur J Obstet Gynecol Reprod Biol 2017;210:69-75.
16. Tanaka T, Hayashi H, Kutsuzawa T, et al. Treatment of interstitial ectopic pregnancy with methotrexate: Report of a successful case. Fertil Steril 1982;37:851-852.
17. Olofsson JI, Poromaa IS, Ottander U, et al. Clinical and pregnancy outcome following ectopic pregnancy; a prospective study comparing expectancy, surgery and systemic methotrexate treatment. Acta Obstet Gynecol Scand 2001;80:744-749.
18. Menon S, Colins J, Barnhart KT. Establishing a human chorionic gonadotropin cutoff to guide methotrexate treatment of ectopic pregnancy: A systematic review. Fertil Steril 2007;87:481-484.
19. Peng B, Klausen C, Campbell L, Leung PCK. Gonadotropin-releasing hormone and gonadotropin-releasing hormone receptor are expressed at tubal ectopic pregnancy implantation sites. Fertil Steril 2016;105:1620-1627.e3.
20. Ali SA. The aromatase inhibitor and Gnrh antagonist versus methotrexate for management of undisturbed ectopic pregnancy. NIH: U.S. National Library of Medicine 2020. Accessed 23 June 2020. https://clinicaltrials.gov/ct2/show/NCT04308343.
21. Cohen A, Almog B, Satel A, et al. Laparoscopy versus laparotomy in the management of ectopic pregnancy with massive hemoperitoneum. Int J Gynecol Obstet 2013;123:139-141.
22. Lagana AS, Vitale SG, De Dominici R, et al. Fertility outcome after laparoscopic salpingostomy or salpingectomy for tubal ectopic pregnancy A 12-years retrospective cohort study. Ann Ital Chir 2016;87:461-465.
23. Mol F, van Mello NM, Strandell A, et al. Salpingotomy versus salpingectomy in women with tubal pregnancy (ESEP study): An open-label, multicentre, randomised controlled trial. Lancet 2014;383(9927):1483-1489.
24. Bangsgaard N, Lund CO, Ottesen B, Nilas L. Improved fertility following conservative surgical treatment of ectopic pregnancy. BJOG 2003;110:765-770.
25. Chen L, Zhu D, Wu Q, Yu Y. Fertility outcomes after laparoscopic salpingectomy or salpingotomy for tubal ectopic pregnancy: A retrospective cohort study of 95 patients. Int J Surg 2017;48:59-63.
26. Hackmon R, Sakaguchi S, Koren G. Effect of methotrexate treatment of ectopic pregnancy on subsequent pregnancy. Can Fam Physician 2011;57:37-39.
27. Irani M, Robles A, Gunnala V, Spandorfer SD. Unilateral salpingectomy and methotrexate are associated with a similar recurrence rate of ectopic pregnancy in patients undergoing in vitro fertilization. J Minim Invasive Gynecol 2017;24:777-782.
Ms Ranchal is a fourth-year medical student at the University of British Columbia. Dr Dunne is a clinical assistant professor at the University of British Columbia and a co-director at the Pacific Centre for Reproductive Medicine in Vancouver. She also serves on the BCMJ Editorial Board.
