Sexual lubricants: Practice tips
Considerations for making well-informed, tailored recommendations to patients.
According to the World Health Organization, sexual health promotion is “fundamental to the overall health and well-being of individuals, couples, and families, and to the social and economic development of communities and countries.”[1] In the realm of sexual health and general self-care, lubricants are often recommended to patients to aid with overall sexual satisfaction and to enhance pleasure, especially in the context of self-stimulation, anal play/intercourse, and dyspareunia, or, in patients with vulvas/vaginas, genitourinary syndrome of menopause.[2] Despite this, patients often don’t know which lubricants to use; commonly disclose that they feel shy about asking; and often use lubricants that may cause or worsen irritation or that may not be compatible with barrier contraception or sexual play aids, enhancers, and toys.
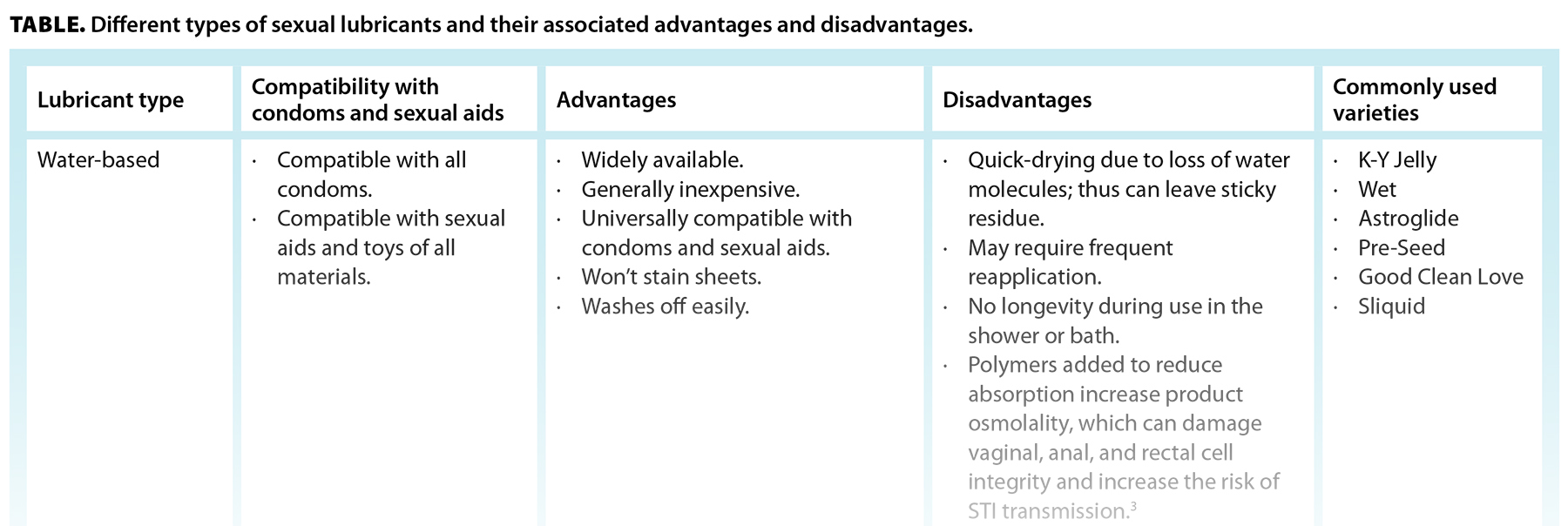
Sexual lubricants are typically liquids or gels designed to reduce friction during sexual activity. There are three types of lubricants available: water-based, silicone-based, and natural oil–based [Table]. In general, products that should not be used as lubricants include baby oil, burn ointment, butter, cooking oil, mineral oil, fish oil, suntan oil, hemorrhoid cream, petroleum jelly, and body or hand lotions.
Vaginal pH is typically between 3.8 and 4.5, while rectal pH is closer to 7.0. Alteration in vaginal pH can lead to vulvovaginitis and is more supportive of HIV survival. According to the World Health Organization, optimally, the osmolality of a water-based lubricant should not exceed 320 mOsm/kg, though a more liberal goal of up to 1200 mOsm/kg is acceptable (note that most of the common, commercially available water-based lubricant products are between 2000 and 6000 mOsm/kg), with a pH between 5.0 and 7.0 for anal intercourse, or around 4.5 if vaginal intercourse is the primary activity.[3] Moreover, some lubricants contain spermicides such as nonoxynol-9—a known mucosal irritant that can increase risk of HIV transmission—or additional ingredients that claim benefits like delaying ejaculation or stimulating effects.[3] These ingredients, including local anesthetics such as benzocaine, are not subject to medical regulation and are often irritants that should be used cautiously, if not avoided altogether. This information, which helps patients make an educated decision when choosing what to put inside their bodies, can be challenging to acquire for many commercially available products. Edwards and Panay’s article “Treating vulvovaginal atrophy/genitourinary syndrome of menopause: How important is vaginal lubricant and moisturizer composition?” contains a table listing the osmolality and pH of some common commercially available water-based lubricants and vaginal moisturizers, which may act as a reference guide.[7]
For most patients, when recommending a lubricant, we consider the types of sexual activities the patient engages in; STI/contraceptive barriers involved; sexual aids or toys involved; and skin conditions, known allergies, or tendencies toward yeast or bacterial vaginosis infections. We recommend avoiding products containing glycerin, parabens, propylene glycol, fragrance, flavor, or special ingredients that claim pleasure-enhancing properties. For many patients, if they’re not using silicone sexual aids, a silicone-based lubricant works well and is generally less irritating and better tolerated. However, water-based lubricants are less expensive and more readily available, and we advise that patients who use silicone-based sexual aids keep a water-based lubricant available. To find a water-based lubricant that is iso-osmolar, we suggest that patients consider water-based lubricants rated as being “fertility-friendly” or that clearly advertise as being “iso-osmolar.” If a person is inclined to use an oil-based lubricant and latex contraceptives are not being used, we typically suggest unrefined or virgin coconut oil, as it is easier to apply due to starting as a solid at room temperature but quickly melting at body temperature. We advise purchasing an unrefined oil and using a spoon to scoop it from the jar to reduce bacterial contamination of the container. Judicious lubricant use during anal activity is also recommended, as the rectum produces no innate lubricant, and thus the mucosa is highly prone to injury from friction. Generally, silicone-based lubricants work well for anal intercourse that does not involve using a silicone toy, and some with thicker consistencies are available on the market.
In some circumstances, however, a sexual lubricant may not be enough to treat a patient’s symptoms. Patients with vulvas/vaginas who present with sexual pain warrant a thorough history and gentle examination, as chronic skin conditions, vaginismus, and provoked vestibulodynia (among other conditions) may be contributing to their discomfort, and lubrication alone is an inappropriate treatment. Consideration for a gynecology or sexual medicine referral should be given in these cases. Moreover, some perimenopausal and postmenopausal patients present having already tried a sexual lubricant yet continue to have dryness related to declining estrogen, which is known as the genitourinary syndrome of menopause. In fact, symptoms of vulvovaginal atrophy affect nearly half of all perimenopausal and postmenopausal individuals.[8] In these instances, ensuring patients use an appropriate lubricant is prudent if their symptoms are limited to sexual activity. However, adding a longer-acting, pH-balanced vaginal moisturizer or topical estrogen (or dehydroepiandrosterone/prasterone) may be paramount to improving their comfort and symptoms if the patients have no contraindications.[7] Typically, hypoestrogenic vulvovaginal tissue will be thin and pale, with loss of vaginal rugae. Sexual discomfort may also occur during breastfeeding or chestfeeding due to a hypoestrogenic vulvovaginal environment, and lubricants and vaginal estrogen should be considered for these patients as well. The bottom line is that when a patient presents with sexual health, reproductive, or genital pain concerns, care providers should ask what type of lubricant they’re using (if any) and be well informed to make tailored suggestions compatible with the patient’s unique sexual functioning situation.
hidden
This article has been peer reviewed.
 |
| This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. |
References
1. World Health Organization. Sexual health. Accessed 22 October 2023. www.who.int/health-topics/sexual-health.
2. Wolfman W, Krakowsky Y, Fortier M. Guideline 422d: Menopause and sexuality. J Obstet Gynaecol Can 2021;43:1334-1341.e1.
3. World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO/UNFPA/FHI360: Advisory note. 2012. Accessed 9 May 2023. https://iris.who.int/handle/10665/76580.
4. World Health Organization. WHO/United Nations Population Fund (UNFPA) specifications for plain lubricants. WHO Drug Inf 2019;33:562-572.
5. National Library of Medicine. Label: Intrarosa—Prasterone insert. DailyMed. Updated 11 November 2020. Accessed 22 October 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=df731acd-7276-4fef-b037-bc7f30c112cb.
6. Medisca. Witepsol® H-15. 2023. Accessed 22 October 2023. www.medisca.com/products/witepsol-h-15.
7. Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: How important is vaginal lubricant and moisturizer composition? Climacteric 2016;19:151-161.
8. Lev-Sagie A. Vulvar and vaginal atrophy: Physiology, clinical presentation, and treatment considerations. Clin Obstet Gynecol 2015;58:476-491.
hidden
Dr Correia is a sexual medicine physician and medical director at the BC Centre for Sexual Medicine, Vancouver Coastal Health; a clinical assistant professor in the Department of Psychiatry at the University of British Columbia; sexuality theme lead of the MD Undergraduate Program at UBC; and an associate member of the Department of Obstetrics and Gynaecology at UBC. Dr Rabicki is a postgraduate year 5 resident in the Department of Obstetrics and Gynaecology at UBC.
Corresponding author: Dr Katherine Rabicki, krabicki@gmail.com.