Are miscarriages more common during the COVID-19 pandemic? An analysis of in vitro fertilization pregnancies in British Columbia
ABSTRACT
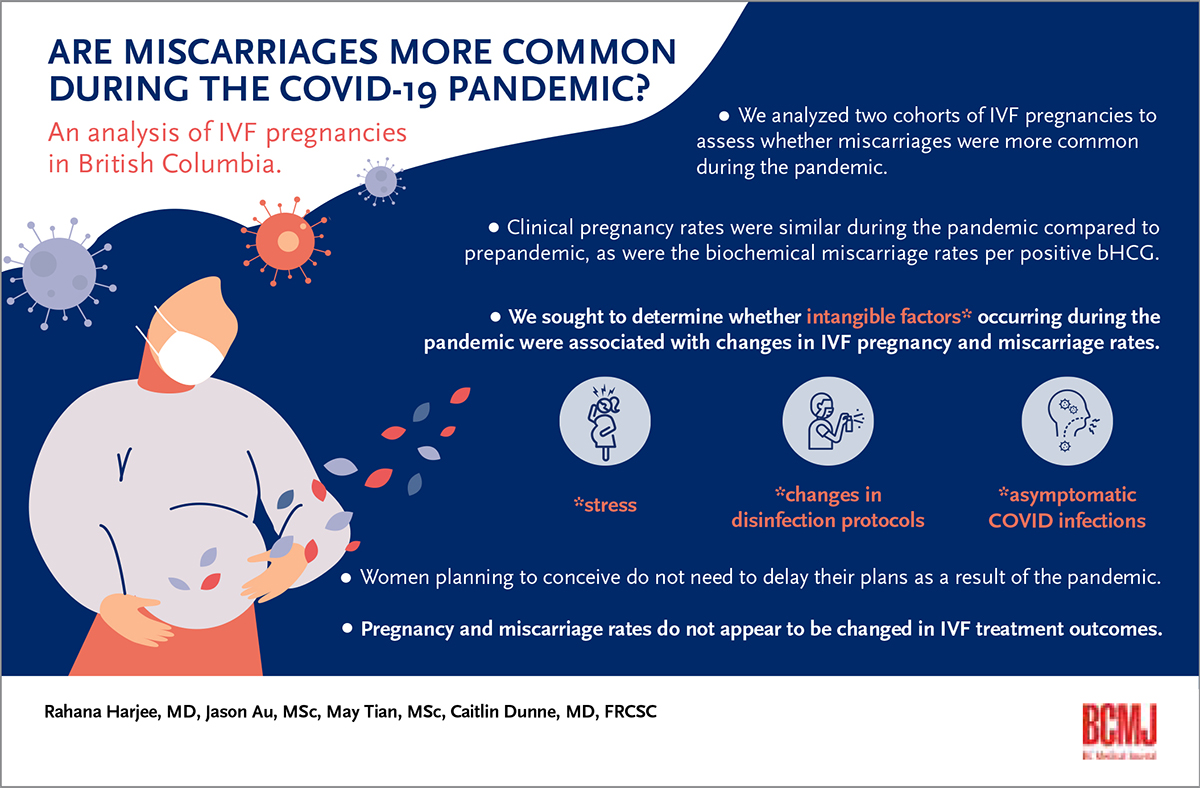
Background: An assessment was conducted to determine whether the ongoing COVID-19 pandemic is associated with increased miscarriage rates in in vitro fertilization pregnancies.
Methods: A retrospective quality assurance analysis with case-matched controls was conducted at a private fertility centre in BC. In vitro fertilization/intracytoplasmic sperm injection cycles between April and December 2020 (during the pandemic) were compared with cycles from April 2018 to March 2020 (prepandemic) to assess differences in pregnancy and miscarriage rates, including fresh transfer cycles, frozen donor egg cycles, and frozen embryo transfer cycles (with and without preimplantation genetic testing). The miscarriage rate was analyzed per pregnancy.
Results: In total, 854 cycles that occurred during the pandemic were compared with 1852 cycles that preceded the pandemic. Patients’ ages were similar between the two groups. The mean number of embryos transferred was similar in the donor egg cycles and frozen embryo transfer cycles (with and without preimplantation genetic testing). Significantly fewer embryos were transferred in the fresh transfer group than in the frozen transfer group (1.36 versus 1.54 [P < 0.0001]), which is likely attributed to a temporal change in practice. Overall, there were no significant differences in clinical pregnancy rates. In all treatment types, the biochemical loss per positive beta-human chorionic gonadotropin and the spontaneous miscarriage rate per clinical pregnancy were not significantly higher during the COVID-19 pandemic than prepandemic, nor was the total loss rate per positive beta-human chorionic gonadotropin.
Conclusions: The COVID-19 pandemic and associated intangible factors do not appear to affect clinical pregnancy rates or miscarriage rates in in vitro fertilization patients.
Women who are planning to conceive do not need to delay their plans due to the COVID-19 pandemic because pregnancy and miscarriage rates do not appear to be affected in in vitro fertilization treatment outcomes.
Background
Individuals have experienced increased stress due to the ongoing COVID-19 pandemic.[1] Higher stress levels can be associated with increased spontaneous miscarriage rates.[2] Pandemic stress may be exacerbated in patients who are undergoing fertility treatments due to numerous factors, including financial strain, anxiety, delays in care, pandemic uncertainty, and advancing age.[3-5] We analyzed two cohorts of in vitro fertilization (IVF) pregnancies to assess whether miscarriages were more common during the pandemic. We sought to determine whether intangible factors such as stress, changes in disinfection protocols, and asymptomatic COVID-19 infections during the pandemic were associated with changes in IVF pregnancy and miscarriage rates.
Methods
A retrospective quality assurance study with case-matched controls was conducted at the Pacific Centre for Reproductive Medicine, a private university-affiliated fertility clinic in British Columbia. Quarterly outcomes assessments are conducted routinely to monitor centre outcomes and provide valuable information to clinicians and patients. IVF cycles between April and December 2020 (during the pandemic) were compared with cycles from April 2018 to March 2020 (prepandemic) to assess for differences in pregnancy and miscarriage rates. Both biochemical miscarriages (human chorionic gonadotropin > 10 IU/L without ultrasound evidence of a pregnancy) and clinical miscarriages (ultrasound evidence of a gestational sac/yolk sac but with no fetal heart activity) were examined.
Four IVF treatment categories were included: fresh embryo transfer cycles, frozen donor egg cycles, and frozen embryo transfer cycles (with and without preimplantation genetic testing). Asymptomatic patients were not tested for COVID-19; however, comprehensive screening was conducted during each clinic visit, in keeping with provincial standards for nonhospital medical surgical facilities. The miscarriage rate was analyzed per pregnancy. Statistics were analyzed using the Student’s t test (continuous variables) and Fisher exact test (proportions).
Results
Nine months of IVF data from the start of the pandemic (854 cycles) were compared with the 24 months immediately preceding the pandemic (1852 cycles). Stratifying by cycle type, patients’ ages were similar between the two groups. The mean number of embryos transferred was similar in the donor egg cycles and frozen embryo transfer cycles (with and without preimplantation genetic testing). Significantly fewer embryos were transferred in the fresh transfer group than in the frozen transfer group (1.36 versus 1.54 [P < 0.0001]), which is likely reflective of a temporal change in practice, which encourages single embryo transfer to reduce twin pregnancies.
The clinical pregnancy rates prepandemic and during the pandemic were similar [Figure 1], as were the biochemical miscarriage rates per positive beta-human chorionic gonadotropin [Figure 2]. Across all treatment types, the spontaneous miscarriage rate per clinical pregnancy was not statistically significantly higher during the COVID-19 pandemic compared to prepandemic. For example, in fresh embryo transfers, clinical miscarriages occurred in 12.2% of patients prepandemic and 12.6% during the pandemic (P > 0.05). Clinical miscarriage rates for frozen embryos (without preimplantation genetic testing) were 10.5% prepandemic and 11.8% during the pandemic (P > 0.05) [Figure 3].
Discussion
As the pandemic evolves, more research on pregnancy is becoming available. Retrospective studies thus far have reported reassuring findings in terms of female fertility, laboratory outcomes, and clinical outcomes in patients undergoing fertility treatment after mild or asymptomatic SARS-CoV-2 infection.[6,7] Wang and colleagues studied 65 women with a positive SARS-CoV-2 antibody result and found similar results when assessing ovarian reserve, ovarian stimulation, and fertilization and blastocyst rates, as well as implantation, pregnancy, and early miscarriage rates compared to matched controls.[6] Orvieto and colleagues assessed IVF outcomes in seven women who had resumed treatment after recovering from COVID-19: there were no significant differences in their cycles when compared to their IVF cycles prior to COVID-19 infection, apart from a reduced proportion of top-quality embryos postinfection (P = 0.03).[7] Rotshenker-Olshinka and colleagues examined 285 women with first-trimester pregnancies (113 during the pandemic; 172 prior to the pandemic) and reported no increases in first-trimester miscarriage in asymptomatic patients during the COVID-19 study period.[8] In September 2021, the New England Journal of Medicine published correspondence regarding 2022 pregnant women who had received a COVID-19 vaccine: 14.1% experienced a miscarriage, which was within the expected range based on historical cohorts.[9]
Our quality assurance analysis of IVF pregnancies demonstrated similar pregnancy and miscarriage rates pre- and mid-pandemic. These results are reassuring to people seeking fertility treatment, many of whom might experience significant age-related oocyte quality decline should they be forced to wait years for pandemic resolution.
A limitation of our study is that none of the patients were vaccinated against COVID-19 because the vaccine was not available yet. We also cannot estimate the rate of asymptomatic infections because, as is the case across BC, patients were not routinely tested for COVID-19 before outpatient surgical procedures.
Data overwhelmingly indicate that COVID-19 infection presents a significantly higher risk to pregnant individuals due to increasing maternal morbidity, mortality, and neonatal complications; in Ontario, 7% to 15% of pregnant patients with moderate to severe infection required hospitalization.[10,11] However, there is good evidence that mRNA vaccines can protect pregnant women from severe COVID-19 symptoms, and the vaccines are safe and recommended for women prepregnancy and during pregnancy.[12]
Conclusions
Our analysis of pre- and mid-pandemic IVF pregnancies indicates that the less tangible effects of the COVID-19 pandemic, including changes in disinfection protocols affecting the baseline volatile organic compounds level, increased stress endured by patients, and possible asymptomatic COVID-19 infection, do not appear to affect clinical pregnancy rates or miscarriage rates in IVF patients. Anyone planning to conceive can be reassured that they do not need to delay their plans as a result of the pandemic because pregnancy and miscarriage rates do not appear to be affected in IVF treatment outcomes.
Competing interests
Dr Dunne is a member of the BCMJ Editorial Board, but did not participate in decision making regarding the acceptance of this article.
This article has been peer reviewed.
 |
| This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. |
References
1. Brotto L, Ogilvie G, Kaida A, et al. Early release: Impact of COVID-19 pandemic controls on mental health outcomes for a provincial population-based cohort through a sex and gender lens. Women’s Health Research Institute, 2021. Accessed 26 January 2022. https://whri.org/wp-content/uploads/2021/02/COVID-19-RESPPONSE_Mental-Health-Report_Feb-18-2021_FINAL.pdf.
2. Qu F, Wu Y, Zhu Y-H, et al. The association between psychological stress and miscarriage: A systematic review and meta-analysis. Sci Rep 2017;7:1731.
3. Kirubarajan A, Patel P, Tsang J, et al. The psychological impact of the COVID-19 pandemic on fertility care: A qualitative systematic review. Hum Fertil (Camb) 2021 Jun 11:1-8.
4. Gupta M, Jaiswal P, Bansiwal R, et al. Anxieties and apprehensions among women waiting for fertility treatments during the COVID-19 pandemic. Int J Gynaecol Obstet 2021;152:441-443.
5. Boivin J, Harrison C, Mathur R, et al. Patient experiences of fertility clinic closure during the COVID-19 pandemic: Appraisals, coping and emotions. Hum Reprod 2020;35:2556-2566.
6. Wang M, Yang Q, Ren X, et al. Investigating the impact of asymptomatic or mild SARS-CoV-2 infection on female fertility and in vitro fertilization outcomes: A retrospective cohort study. EClinicalMedicine 2021;38:101013.
7. Orvieto R, Segev-Zahav A, Aizer A. Does COVID-19 infection influence patients’ performance during IVF-ET cycle?: An observational study. Gynecol Endocrinol 2021;37:895-897.
8. Rotshenker-Olshinka K, Volodarsky-Perel A, Steiner N, et al. COVID-19 pandemic effect on early pregnancy: Are miscarriage rates altered, in asymptomatic women? Arch Gynecol Obstet 2021;303:839-845.
9. Zauche LH, Wallace B, Smoots AN, et al. Receipt of mRNA Covid-19 vaccines and risk of spontaneous abortion. N Engl J Med 2021;385:1533-1535.
10. Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: The INTERCOVID-19 Multinational Cohort Study. JAMA Pediatr 2021;175:817-826.
11. Munshi L, Wright JK, Zipursky J, et al. The incidence, severity, and management of COVID-19 in critically ill pregnant individuals. Science Briefs of the Ontario COVID-19 Science Advisory Table. 2021;2(43).
12. Poliquin V, Castillo E, Boucoiran I, et al. SOGC statement on COVID-19 vaccination in pregnancy. Society of Obstetricians and Gynaecologists of Canada, 2021. Accessed 26 January 2022. https://sogc.org/common/Uploaded%20files/Latest%20News/SOGC_Statement_COVID-19_Vaccination_in_Pregnancy.pdf.
Dr Harjee is a resident physician in the Department of Obstetrics and Gynaecology, University of British Columbia. Mr Au is an embryologist at the Pacific Centre for Reproductive Medicine. Ms Tian is an embryologist at the Pacific Centre for Reproductive Medicine. Dr Dunne is a clinical associate professor in the Department of Obstetrics and Gynaecology, University of British Columbia, and a reproductive endocrinology and infertility specialist at the Pacific Centre for Reproductive Medicine.

