Dr D.S. Fredrickson: Founding father of the field of lipidology
ABSTRACT: Dr D.S. Fredrickson is considered by many to be the founding father of lipidology. An exceptional scientist, Fredrickson discovered Tangier disease and cholesteryl ester storage disease, two genetic conditions caused by aberrant lipid metabolism. His identification of various apolipoprotein components contributed to our current understanding of lipid transport and physiology. His classification of lipoprotein abnormalities, established on the basis of electrophoretic plasma lipoprotein patterns, was accepted by the World Health Organization as a standard of clinical practice and provoked global interest in dyslipidemic disorders.
Fredrickson was part of a remarkable trio of scientists who devised the formula for estimating low density lipoprotein cholesterol levels, a calculation that continues to be used in laboratories worldwide. He was also instrumental in the creation of the Lipid Research Clinics Program, which sponsored the landmark Coronary Primary Prevention Trial, the first major study to definitively demonstrate that lowering cholesterol reduces coronary heart disease risk.
In the 21st century, physicians are well versed in the association between total cholesterol (TC) and coronary heart disease (CHD) risk. The graded relationship of TC and CHD has been meticulously documented in multiple epidemiological studies.[1-3] In particular, the causal role of low density lipoprotein cholesterol (LDL-C) in atherosclerosis is supported by various clinical trials demonstrating concurrent reductions in LDL-C level and CHD morbidity and mortality.[3,4] Lipid-lowering therapies, especially statins, are known to be effective in both primary[5-8] and secondary[9-11] prevention of CHD. Certainly our present understanding of lipids exemplifies the legacy of Dr Donald Sharp Fredrickson, whose pioneering work and inspiring endeavors have been fundamental in lipidology.
The quintessential physician-scientist, Dr Fredrickson contributed to medical breakthroughs that have shaped our understanding of lipid metabolism and pathologies.
Early years
Donald Sharp Fredrickson was born in Canon City, Colorado, in 1924 to an attorney father. He commenced undergraduate studies at the University of Colorado, and subsequently transferred to the University of Michigan, where he graduated with a bachelor of science degree in 1946 and an MD with distinction in 1949. Under the mentorship of George Thorn at Boston’s Peter Bent Brigham Hospital (now the Brigham and Women’s Hospital), Fredrickson completed a residency and fellowship in internal medicine. He then spent a year in the laboratory of Ivan Frantz, a cholesterol biochemist, at the Massachusetts General Hospital.
In 1953 Fredrickson moved to Bethesda, Maryland, and the National Heart Institute (a division of the National Institutes of Health, which was renamed the National Heart, Lung, and Blood Institute in 1976). There he began a tremendously fruitful career in lipidology. At the institute Fredrickson furthered his research in lipids and lipoproteins with future Nobel laureate Christian B. Anfinsen; dabbled in cholesterol metabolism with Daniel Steinberg; and worked with collaborators to isolate and sequence the apolipoprotein components A-II, C-I, C-II, and C-III.
Together with John Stanbury and James Wyngaarden, Fredrickson also edited The Metabolic Basis of Inherited Disease, a compendium of information on hereditary disorders first published in 1960. The four-volume work, currently in its eighth edition, has since been renamed The Metabolic and Molecular Bases of Inherited Disease, and continues to be recognized as a classic in molecular medicine.
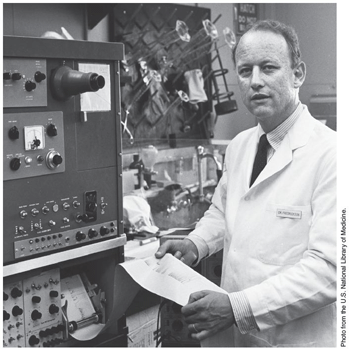
Dr D.S. Fredrickson, shown here in 1969.
Tangier disease and cholesteryl ester storage disease
During the 1960s and 1970s Fredrickson and his colleagues were revered for their discovery of two familial lipid disorders. The first, Tangier disease (TD), was named after the island in Chesapeake Bay where the original kindred was identified. TD manifested clinically with hepatosplenomegaly, peripheral neuropathy, and tonsils “of unusual size and coloration.”[12] Biochemically, a significant decrease in high-density-lipoprotein cholesterol (HDL-C) was evident.
The hereditary defect responsible would be identified simultaneously in Europe and in Vancouver, Canada, 40 years later—mutation of the ATP-binding cassette transporter A1 (ABCA1) gene, encoding for the cholesterol efflux regulatory protein, on chromosome 9q31.[13-15] Impaired cholesterol efflux from macrophages gives rise to foam cells, and may potentially explain the premature CHD frequently noted in TD patients.[15] Diminished efflux may also lead to lipid-depleted nascent HDL particles, which are quickly catabolized.[16]
The second discovery made by Fredrickson and his colleagues, cholesteryl ester storage disease (CESD), is an autosomal recessive condition attributed to mutation of the gene for encoding for lysosomal acid lipase (LAL) on chromosome 10q23.2-q23.3.[17,18] Normally, when LDL binds to its receptor and undergoes endocytosis, the enzyme LAL hydrolyzes and releases cholesterol from the lysosomes.[18]
The subsequent rise in intracellular cholesterol acts by way of negative feedback and reduces expression of both LDL receptor and hydroxymethylglutaryl-CoA reductase (HMG-CoA reductase), thereby maintaining cholesterol homeostasis.[18]
Not surprisingly, LAL deficiency leads to cholesteryl ester aggregation, upregulation of LDL receptor and HMG-CoA reductase, and further accumulation of lysosomal lipids. Complete absence of LAL activity is seen in Wolman disease, and affected patients rarely survive beyond 6 months of age.[19] In contrast, partial LAL deficiency accounts for CESD, with a patient life expectancy of less than 30 years.[20]
CESD can present in childhood or adulthood with liver abnormalities ranging from elevated transaminases to hepatomegaly, jaundice, fibrosis, and liver failure.[18] Hypercholesterolemia (and oftentimes hypertriglyceridemia), decreased HDL-C, and premature atherosclerosis are also features of this disease.[18]
Fredrickson classification of hyperlipidemias
Fredrickson’s best-known contribution to lipidology is his classification of lipoprotein disorders (Table), developed with Robert Levy and Robert Lees. The five phenotypes were described according to their clinical features and plasma lipoprotein patterns on paper electrophoresis.[21] This classification system was the first to recognize phenotype III (now known as familial dysbetalipoproteinemia) as a unique clinical and biochemical entity distinct from the heterogeneous conditions formerly referred to as familial hypercholesterolemia.[22-24]
It is important to note that Fredrickson’s classification is based simply on biochemical parameters, and does not represent actual diagnoses or address the source of dyslipidemia as primary or secondary. Furthermore, it does not take into account plasma HDL-C levels. Nevertheless, it was adopted by the World Health Organization as an international standard in 1972,[25,26] and thus brought global attention to lipoprotein and lipid disorders. Amid the recent advances in molecular medicine, the Fredrickson classification is increasingly being replaced by a categorization scheme that organizes lipoprotein abnormalities on the basis of their genetic etiology and pathophysiology.
The Lipid Research Clinics Coronary Primary Prevention Trial
Fredrickson was pivotal in the establishment of the nationwide Lipid Research Clinics Program at the National Heart Institute.[26] The group was responsible for the landmark Coronary Primary Prevention Trial (CPPT),[27,28] the first major study to conclusively demonstrate the efficacy of cholesterol lowering in reducing CHD.[29,30] When study enrolment began in 1973, observational epidemiological studies had shown an association between raised TC/LDL-C and increased CHD. However, no clinical trials had yet provided strong evidence for the causal role of these lipids in the pathogenesis of CHD.
The results of these observational studies, though encouraging, were inconclusive because of insufficient sample size, inadequate cholesterol lowering, lack of a double-blind design, inability to achieve identical study groups, and problematic statistical analysis.[27]
The multicentre, randomized, double-blind CPPT study followed 3806 asymptomatic middle-aged males with primary hypercholesterolemia for a mean period of 7.4 years. The study subjects were divided into two groups: the treatment group received 24 g of the bile acid sequestrant cholestyramine daily, while the control group received a placebo. Subjects in the cholestyramine arm demonstrated a 19% decrease (P < .05) in the combined primary endpoint of definite CHD death and/or definite nonfatal myocardial infarction when compared with subjects in the control arm. Separate analysis of the resin-treated group revealed that a 19% decline in CHD risk was associated with each decrement of 8% in total cholesterol level or 11% in LDL-C level (P < .001).
Equipped with CPPT’s definitive and long-sought-after evidence for CHD risk reduction by way of cholesterol lowering, the National Institutes of Health launched the National Cholesterol Education Program (NCEP) in November of 1985.[29] Through its educational campaigns and expert panel recommendations, such as the Adult Treatment Panel III guidelines,[4] the NCEP has taken a leading role in the diagnosis and management of hypercholesterolemia.
The Friedewald-Levy-Fredrickson formula
Fredrickson, in conjunction with Robert Levy and William Friedewald, devised an equation for estimating LDL-C in 1972.[31] This method, now commonly known as the Friedewald formula, uses fasting lipid levels to calculate a value for low density lipoprotein cholesterol:
LDL-C = TC 2 HDL-C 2 TG/2.2 (in units of mmol/L)
or
LDL-C = TC 2 HDL-C 2 TG/5 (in units of mg/dL)
In this formula, where TG stands for triglycerides, and TG/2.2 (or TG/5) serves as a proxy for very low density lipoprotein cholesterol (VLDL-C), the ratio of the mass of triglyceride to that of cholesterol in VLDL is assumed to be relatively constant. An LDL-C value can therefore be obtained indirectly using the total cholesterol, HDL-C, and triglyceride levels, all of which can be quantitated with routine analyses and rapid lipoprotein precipitation. This consequently eliminates the need to measure LDL-C directly using ultracentrifugation—a cumbersome, costly, and not widely available process. Although convenient, the Friedewald formula has several well-established limitations,31 and should not be used when specimens indicate:
• Triglyceride concentration above 4.52 mmol/L (400 mg/dL).
• Presence of chylomicrons.
• Type III hyperlipoproteinemia (due to beta-VLDL particles).
• Presence of other cholesterol-rich particles (e.g., lipoprotein(a), lipoprotein-X).
Executive engagements
In addition to his research involvements and clinical duties, Fredrickson took on prominent administrative positions as director of the National Heart Institute from 1966 to 1968, then as director of the National Institutes of Health from 1975 to 1981. During his tenure at the NIH, the rise of recombinant DNA technology led to political and ethical controversies. Fredrickson’s wisdom and foresight during this tumultuous period shaped public policies that maintained a delicate balance between the pursuit of scientific knowledge and social responsibility.
From 1984 to 1987 Fredrickson was president of the Howard Hughes Medical Institute, a leading private medical research and funding establishment. From 1987 until his death in 2002 Fredrickson was a scholar-in-residence at the National Library of Medicine.
Summary of achievements
Fredrickson was an internationally renowned expert on lipid metabolism disorders. His discovery of the apolipoprotein components and hereditary cholesterol diseases greatly enriched our knowledge of lipid and lipoprotein physiology. His classification of hyperlipidemias and the subsequent adoption of this system by the World Health Organization helped shine a global spotlight on dyslipidemic conditions. His contribution to the Friedewald formula gave us a simple and inexpensive method to monitor the atherogenic LDL-C marker in our patients.
Fredrickson’s achievements were key to the genesis of lipidology, an entirely unheard of specialty 50 years ago. As a dedicated mentor to the specialty’s first disciples and a contributor to efforts that conclusively demonstrated the link between hyperlipidemia and coronary heart disease, Fredrickson has left a lasting imprint in the field of cardiovascular medicine.
Competing interests
None declared.

