Review Articles
Implementation of human papillomavirus primary screening for cervical cancer in BC
ABSTRACT: In January 2024, British Columbia began a transition from cytology to human papillomavirus testing for cervical cancer screening. Human papillomavirus testing has a higher sensitivity for identifying patients with cervical precancer (cervical intraepithelial neoplasia grade 2 or worse) and cancer. Testing can be completed on either liquid-based samples collected from the cervix by a health care provider during a speculum exam or a sample from the vagina collected using a dry FLOQSwab by either a patient or a health care provider. Self-collected samples have similar accuracy to provider-collected samples and reduce many historical barriers to cervix screening. The implementation of human papillomavirus screening offers sample collection choice to patients and providers and is expected to improve access and equity in cervix screening.
Human papillomavirus primary screening can identify those at risk for cervical precancer and cancer earlier and better than cytology.
British Columbia has been a pioneer in population-based cervical cancer screening due to the initiation of the first cervix screening program in the world in 1955.[1] Since the implementation of cervix screening, the incidence of cervical cancer in BC has decreased by more than 70%.[1] Prior to the availability of cervix screening, 28.4 in 100 000 women were diagnosed with cervical cancer annually.[1] The 2020 incidence rate in BC was 8.1 in 100 000.[2]
The World Health Organization has identified the elimination of cervical cancer as a global public health goal. However, the reduction in cervical cancer incidence in BC has plateaued due to the limited sensitivity of cytology as a screening test and the ongoing challenge with improving the participation rate.
To overcome these factors, BC’s Cervix Screening Program began a transition to human papillomavirus (HPV) primary screening in January 2024. People now have a choice in how they would like to receive cervix screening. HPV primary screening allows for health care provider–collected or patient-collected samples. The screening detects high-risk (oncogenic) genotypes of HPV and thus identifies people more at risk of having cervical dysplasia. Persistent high-risk HPV infections, over 15 to 20 years, cause 99.7% of cervical cancers.[3]
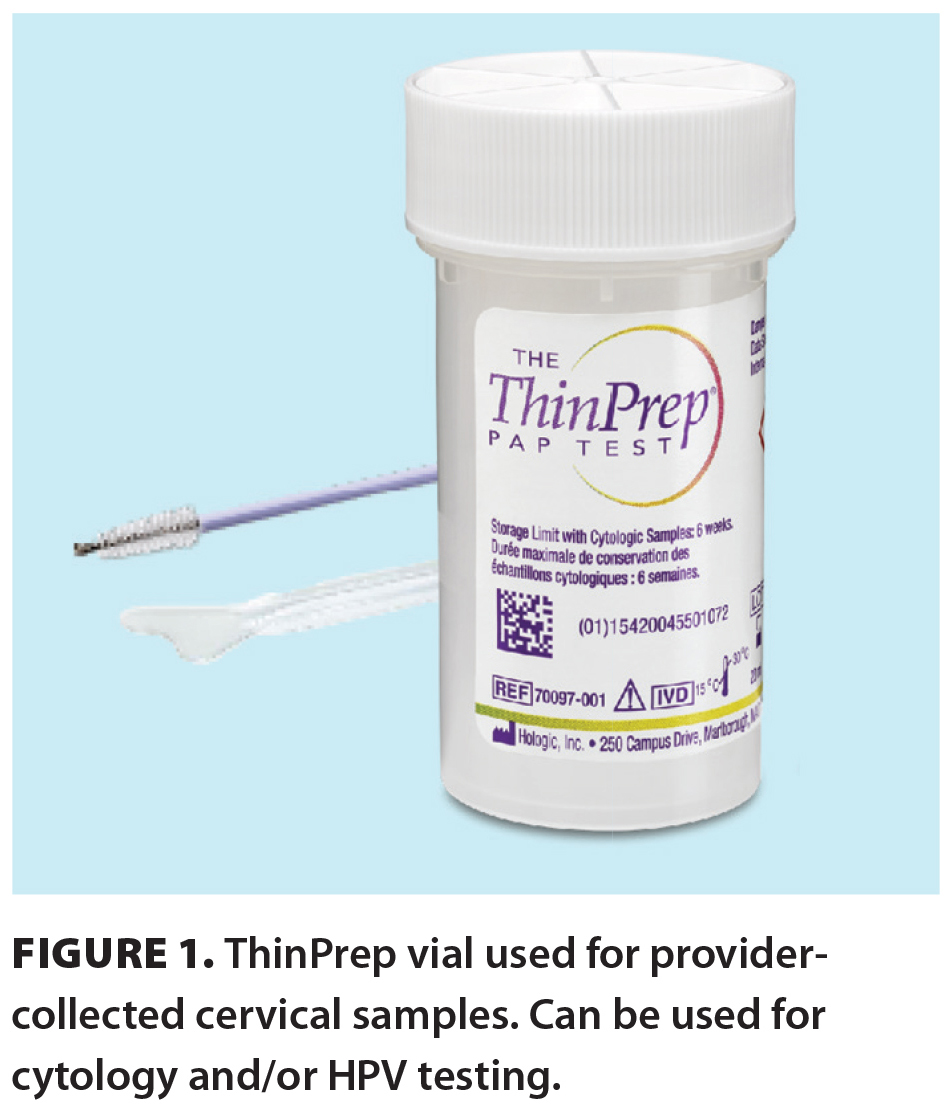 HPV testing can be done on liquid-based cytology samples containing cervical cells collected by a health care provider during a speculum exam and transferred into PreservCyt (ThinPrep vials) [Figure 1]. Patients or providers can use a red-capped Copan dry FLOQSwab for vaginal collection that is then eluded into PreservCyt at the laboratory. Both collection types are tested using DNA amplification by polymerase chain reaction using the Roche Diagnostics cobas HPV assay, which provides information on the presence of genotypes HPV 16 and HPV 18 and pooled results for 12 other high-risk HPV genotypes (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68). HPV 16 and HPV 18 are known to cause 70% of cervical cancers. The cobas HPV assay uses beta-globin found in human DNA as an internal control to indicate sufficient sample collection. Results are reported as invalid if the internal control is absent.
HPV testing can be done on liquid-based cytology samples containing cervical cells collected by a health care provider during a speculum exam and transferred into PreservCyt (ThinPrep vials) [Figure 1]. Patients or providers can use a red-capped Copan dry FLOQSwab for vaginal collection that is then eluded into PreservCyt at the laboratory. Both collection types are tested using DNA amplification by polymerase chain reaction using the Roche Diagnostics cobas HPV assay, which provides information on the presence of genotypes HPV 16 and HPV 18 and pooled results for 12 other high-risk HPV genotypes (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68). HPV 16 and HPV 18 are known to cause 70% of cervical cancers. The cobas HPV assay uses beta-globin found in human DNA as an internal control to indicate sufficient sample collection. Results are reported as invalid if the internal control is absent.
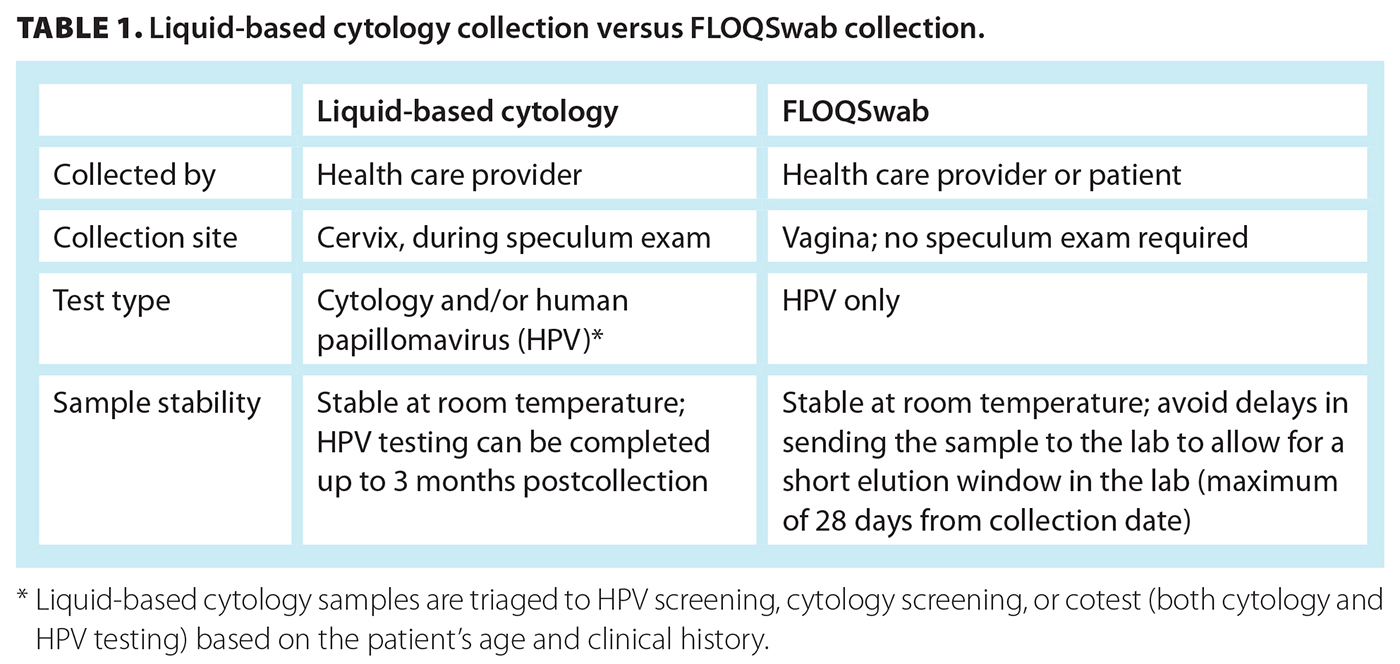
Unlike cytology, HPV testing does not require a sample of cells from the squamocolumnar junction of the cervix; therefore, it can be conducted on vaginal samples collected by either the health care provider or the patient. Self-collected samples have been shown to have similar accuracy to provider-collected samples.[4] In BC, self-screening is available to all age-eligible cervix screening participants. Provider-collected liquid-based cytology samples are triaged at the laboratory to HPV testing, cytology testing, or both HPV and cytology testing (cotest) based on the patient’s screening history and age. A step-down approach to phase out primary cytology screening is in progress. Currently, liquid-based cytology samples are triaged to HPV testing for patients age 55 years and older to ensure they have at least one high-quality screening before aging out of screening. The age for triaging samples to HPV testing may continue to decrease as BC progresses through the transition. Once the transition to HPV primary screening is complete, all liquid-based cytology samples will undergo HPV testing when received at the laboratory. Table 1 shows a comparison of test collection and testing options for liquid-based cytology and dry FLOQSwab vaginal collection.
HPV testing: Improved accuracy
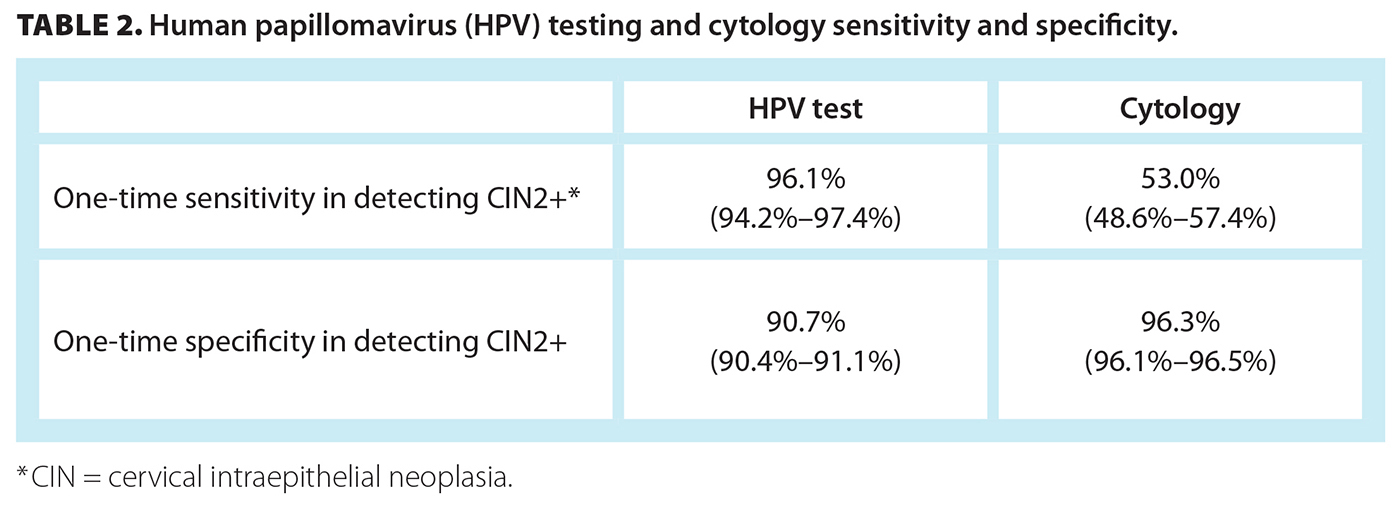
HPV testing is superior to cytology for detecting cervical precancer (cervical intraepithelial neoplasia [CIN] grade 2 or worse) and cancer. HPV screening has a one-time sensitivity for detecting CIN grade 2 or worse (CIN2+) of 96.1%, versus 53.0% for cytology,[5] and a Cochrane review showed a lower likelihood of missing cases of CIN2+ and CIN3+ compared with cytology[6] [Table 2]. The HPV FOCAL trial conducted between 2008 and 2016 determined that, compared with cytology, HPV testing resulted in a significantly lower likelihood of CIN2+ and CIN3+ four years after testing,[7] consistent with other studies in the literature.[8-10] Moreover, HPV FOCAL trial participants have been followed for 10 years or more, and the risk of CIN2+ detection has remained low across all age groups for at least 7 years, thus highlighting the high negative predictive value.[11,12] The improved sensitivity and improved negative predictive value of HPV testing support extending the interval between routine screening to every 5 years.[12,13]
Reducing barriers to screening
HPV testing can be collected cervically or vaginally by a health care professional or vaginally by the patient, which reduces many historical barriers to cervix screening. Several approaches have been implemented over the years to improve reach and participation in screening among underserved populations, including partnering with the First Nations Health Authority to promote community-based screening awareness, conducting engagement at temples and other cultural centres, and partnering with non–health care settings such as salons, but the participation rate for cervix screening has slowly declined to less than the 70% provincial target. Participation in cervix screening is not evenly distributed across populations or cultures: some populations are less likely than others to participate. Factors contributing to the inequity in care are multifactorial, and barriers are both personal and systemic. Cervix screening rates are known or suspected to be lower for the following populations:[14-21]
- Low income.
- Immigrant.
- Indigenous (First Nations, Métis, and Inuit).
- Transgender, gender diverse, and nonbinary.
- Those who are not attached to a primary care provider.
- Rural and remote communities.
- Those who are less familiar with the BC health care system.
- Those who do not speak the language in which service information is available.
- Those with a history of trauma and/or violence.
A history of trauma, cultural barriers, not having a primary care provider, and having difficulty getting to an appointment (e.g., taking time off work, child care needs, distance to clinic) are all known barriers to cytology screening. The ability of patients to collect their own sample at home or wherever they feel most comfortable offers, for the first time, the opportunity to remove many of these barriers.
In a meta-analysis of 56 accuracy studies, the clinical sensitivity of self-collected HPV samples was equivalent to clinician-collected samples for the detection of CIN2+ and CIN3+, for polymerase chain reaction–based assays.[22] These results were confirmed in a subsequent randomized trial that compared physician-collected samples to patient-collected samples.[23] In a systematic review and meta-analysis, participants reported that they would prefer self-sampling over health care provider HPV testing, citing factors such as ease and privacy.[24] This provides confidence that we can safely offer an acceptable alternative collection method for cervix screening in BC. Effectively, patients and health care providers now have a choice for cervix screening.
Eligibility
Screening is recommended for people with a cervix (including women and Two-Spirit, transgender, and gender-diverse individuals), aged 25 to 69 years, who are or have been sexually active. In BC, the next recommended time to screen is provided on the screening test laboratory report from the Cervical Cancer Screening Laboratory. Obtaining the last screening test report from the laboratory is the most accurate way to know if someone is due to screen again. Generally, patients are due to screen if their previous result was:
- Negative for intraepithelial lesion or malignancy cytology result more than 36 months prior.
- HPV-negative more than 60 months prior.
- Low-grade cytology (atypical squamous cells of undetermined significance [ASCUS] or low-grade squamous intraepithelial lesion [LSIL]) more than 6 months prior.
 Patients with a history of CIN2+ and immunocompromised patients may undergo earlier screening based on recommendations from the colposcopist or laboratory based on the patient’s history.
Patients with a history of CIN2+ and immunocompromised patients may undergo earlier screening based on recommendations from the colposcopist or laboratory based on the patient’s history.
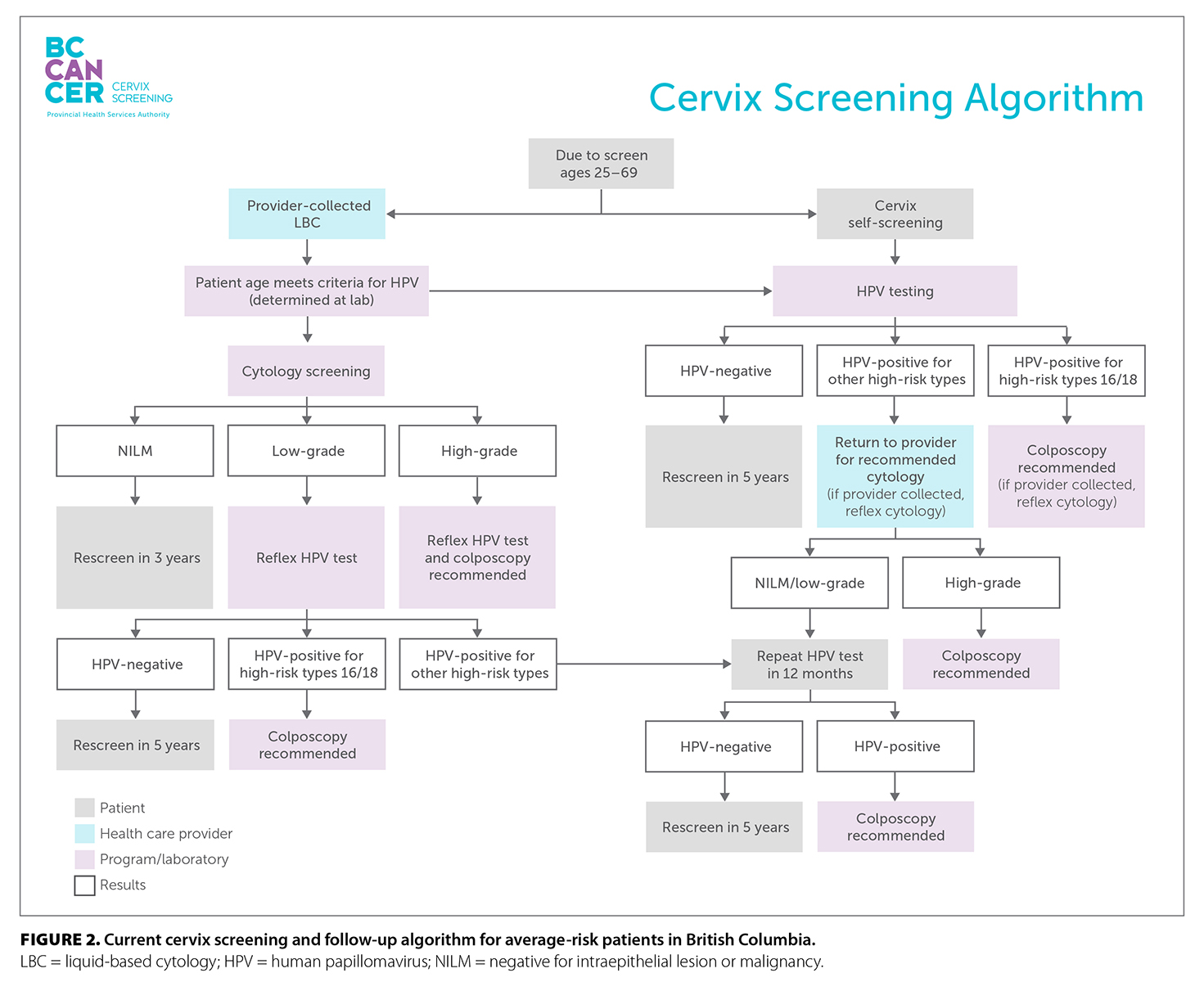
Most patients are eligible to self-screen if they prefer. In some circumstances, the patient’s clinical history would indicate a health care provider–collected liquid-based cytology sample for cotesting. Detailed information on nonaverage risk screening recommendations and follow-up after receiving screening test results is available in the BC Cancer Cervix Screening Program: Program Overview. Figure 2 shows the current screening and follow-up algorithm for average-risk patients.
Importance of providers in cervix screening
Some patients may not be up to date with cervix screening and may have been hesitant to have a pelvic exam. However, the availability of cervix self-screening now provides patients with another choice. Identifying underserved patients and offering self-screening can increase access to care.
Cervix screening is effective only when patients with positive results are willing and able to access follow-up services and treatment. Patients who are willing to self-screen may still be hesitant for follow-up with a specialist for colposcopy. Counseling patients who are recommended for colposcopy to support them in understanding their positive HPV test result and the importance of follow-up testing and to explain what will happen at the time of the colposcopy procedure have been shown to significantly increase rates of adherence to follow-up.[25,26]
Determining the concerns patients have about follow-up procedures and providing support from a trusted care provider are also important aspects of adherence to follow-up. Some patients may benefit from multiple appointments to discuss follow-up and what to expect. Informing patients that colposcopy clinics are often high-volume procedure areas and that they may interact with clerks, nurses, and colposcopists can help patients prepare for the follow-up environment. Patients can also be advised that they can bring a support person with them to the procedure, they have the right to ask questions and seek more information prior to the procedure, and they can ask to stop or pause the exam at any time if they need a break or feel they need to return on another day.
Several patient resources are available, in multiple languages, including brochures, explanatory videos, and patient stories about their experience with colposcopy (www.screeningbc.ca/health-professionals).
Unattached patient process
In January 2024, a provincial unattached patient process was implemented to offer cervix self-screening to eligible people who do not have a health care provider. Legislative and regulatory changes were made to designate the Cervix Screening Program as a “prescribed person.” Patients with a negative HPV test result are provided with their results and are advised to rescreen in 5 years and see a health care provider if they have any symptoms, even if their screening test is negative. Patients who are recommended for follow-up are linked with a clinic in their community to access cytology follow-up testing, speak with a health care provider about their results, and/or obtain support to feel comfortable accessing colposcopy.
Across BC, the Divisions of Family Practice worked with local clinics and health care providers to identify a clinic or provider for unattached patients with positive screen results in each of the 219 Community Health Service Areas in BC. Thanks to the support and creativity of the Divisions of Family Practice, this offers local solutions and community-based care for residents if their screening test results indicate further follow-up is needed. This linking process is the first of its kind in BC and has enabled the Cervix Screening Program to provide an automated, scalable, and sustainable process for population-based screening.
Early outcomes of implementation
From 1 February to 30 June 2024, the following were observed in the program:
- 60 065 cervix self-screening kits were mailed to patients.
- 11 004 of those patients had never been screened in BC.
- 25 154 HPV self-screens were conducted.
- 4680 of those patients had never been screened in BC.
- 19 776 HPV tests on liquid-based cytology-collected samples were conducted.
- 50% of cervix screening transitioned from cytology to HPV testing.
- 4400 patients used the unattached patient process to request and return screening kits.
- 1100 health care provider offices asked to have self-screening swabs available for patients.
Common questions about the transition to HPV screening are addressed in the Box.
Conclusions
HPV primary screening can identify those at risk for cervical precancer and cancer earlier and better than cytology. In addition, HPV primary screening offers innovative approaches to screening to reduce access barriers for equity-deserving groups. Together, these two changes in cervix screening will further reduce the incidence of cervical cancer in BC to less than 4 in 100 000, which will meet the World Health Organization’s criteria for eliminating the disease. More importantly, they are expected to improve equity in screening by reaching vulnerable and underserved populations.
Competing interests
None declared.
BOX. Common questions about the transition to HPV screening.
Is human papillomavirus (HPV) testing done on liquid-based cytology samples?
HPV testing and cytology testing can both can be completed on liquid-based cytology samples. Samples received in the laboratory are triaged to HPV, cytology, or both (cotest) depending on the patient’s age and clinical history. The transition to HPV testing of liquid-based cytology samples started with anyone age 55 years or older who had completed HPV testing. The age for HPV primary screening with liquid-based cytology samples may decrease over time as the province transitions fully to HPV testing.
Do a liquid-based cytology sample and an HPV vaginal swab both need to be collected for patients that have an indication for a cotest, with both cytology and HPV testing required?
A provider should never submit both a liquid-based cytology sample and a vaginal swab for the same patient. If cotesting is indicated, only the liquid-based cytology test should be conducted. The liquid-based cytology sample can be used for both cytology and HPV testing.
Can a patient with a previous atypical squamous cells of undetermined significance (ASCUS) or low-grade squamous intraepithelial lesion (LSIL) result complete self-screening, or is a health care provider–collected sample required?
Patients with a previous ASCUS or LSIL result can complete vaginal self-screening. Most often, patients will be recommended to return to routine HPV screening every 5 years if their result is negative after having a previous ASCUS or LSIL result. Occasionally, the laboratory may recommend a liquid-based cytology collection, depending on the number of previous ASCUS or LSIL results and the length of time since the last abnormal cytology result.
Should clinics wait to have several vaginal self-swabs before returning completed swabs to the laboratory?
It is important to return collected self-screening swabs to the laboratory every 1 to 2 days to reduce the time between collection and when the sample can be eluded into PreservCyt. This can reduce the testing invalid rate.
How can my clinic get access to vaginal self-swab devices to offer in the clinic?
Use the Cervical Cancer Screening Laboratory online order system to request liquid-based cytology supplies and vaginal swabs. This is the same system that is used to order liquid-based cytology (i.e., Pap test) supplies.
Why are patients with a test result of negative HPV and ASCUS or LSIL cytology recommended for screening in 5 years?
In patients with ASCUS who are HPV-negative, the risk for CIN2+ is very low, similar to those with a negative cytology and HPV test. The absolute risk for CIN3 for women with ASCUS and negative HPV is 0.54% at 5 years; therefore, it is recommended to return to routine cervical screening.[8,27]
What does an invalid HPV test result mean?
Samples with insufficient cellularity will yield an invalid result, and recollection will be required. Insufficient cellularity could be due to inadequate time spent during collection or could be related to cells being compromised on the swab prior to elution in PreservCyt in the laboratory. Rotate the swab slowly for 20 seconds to ensure sufficient sample is collected.
Why was cytology not completed on the liquid-based cytology sample I submitted?
The usual reason is that the patient was 55 years of age or older and had a negative HPV screen; therefore, cytology would not be reported in addition to the HPV test result. Cytology and HPV results are both reported only if a reflex cytology was indicated due to a positive HPV screen, if a reflex HPV test was indicated due to an abnormal cytology screen, or if a cotest was indicated. The following are indications for a cotest:
- Following CIN2 or CIN3 excisional treatment and discharge from colposcopy, the patient should have one negative cotest prior to returning to HPV screening every 3 years.
- Following adenocarcinoma in situ excisional treatment and discharge from colposcopy, the patient should have a cotest every 3 years until 69 years of age.
- Following adenocarcinoma in situ excisional treatment, immunocompromised, and discharge from colposcopy, the patient should have a cotest every year until 74 years of age.
- Following total hysterectomy and a history of CIN2, CIN3, or adenocarcinoma in situ, the patient should have a negative cotest prior to discontinuing cervix screening.
This article has been peer reviewed.
 |
| This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. |
References
1. Anderson GH, Boyes DA, Benedet JL, et al. Organisation and results of the cervical cytology screening programme in British Columbia, 1955-85. Br Med J (Clin Res Ed) 1988;296:975-978.
2. BC Cancer. BC Cancer Cervix Screening 2021 program results. Vancouver; 2024.
3. World Health Organization. Cervical cancer. Accessed 15 August 2024. www.who.int/health-topics/cervical-cancer.
4. Arbyn M, Castle PE, Schiffman M, et al. Meta-analysis of agreement/concordance statistics in studies comparing self- vs clinician-collected samples for HPV testing in cervical cancer screening. Int J Cancer 2022;151:308-312.
5. Cuzick J, Clavel C, Petry K-U, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer 2006;119:1095-1101.
6. Koliopoulos G, Nyaga VN, Santesso N, et al. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev 2017;8:CD008587.
7. Ogilvie GS, Krajden M, van Niekerk D, et al. HPV for cervical cancer screening (HPV FOCAL): Complete round 1 results of a randomized trial comparing HPV-based primary screening to liquid-based cytology for cervical cancer. Int J Cancer 2017;140:440-448.
8. Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: A population-based study in routine clinical practice. Lancet Oncol 2011;12:663-672.
9. Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: Final results of the POBASCAM randomised controlled trial. Lancet Oncol 2012;13:78-88.
10. Mayrand M-H, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007;357:1579-1588.
11. Gottschlich A, Hong Q, Gondara L, et al. Evidence of decreased long-term risk of cervical precancer after negative primary HPV screens compared with negative cytology screens in a longitudinal cohort study. Cancer Epidemiol Biomarkers Prev 2024;33:904-911.
12. Gottschlich A, Gondara L, Smith LW, et al. Human papillomavirus-based screening at extended intervals missed fewer cervical precancers than cytology in the HPV For Cervical Cancer (HPV FOCAL) trial. Int J Cancer 2022;151:897-905.
13. Rebolj M, Cuschieri K, Mathews CS, et al. Extension of cervical screening intervals with primary human papillomavirus testing: Observational study of English screening pilot data. BMJ 2022;377:e068776.
14. Woods RR, McGrail KM, Kliewer EV, et al. Breast screening participation and retention among immigrants and nonimmigrants in British Columbia: A population-based study. Cancer Med 2018;7:4044–4067.
15. Demers AA, Decker KM, Kliewer EV, et al. Mammography rates for breast cancer screening: A comparison of First Nations women and all other women living in Manitoba, Canada, 1999-2008. Prev Chronic Dis 2015;12:E82.
16. Lofters A, Glazier RH, Agha MM, et al. Inadequacy of cervical cancer screening among urban recent immigrants: A population-based study of physician and laboratory claims in Toronto, Canada. Prev Med 2007;44:536-542.
17. Lofters AK, Hwang SW, Moineddin R, Glazier RH. Cervical cancer screening among urban immigrants by region of origin: A population-based cohort study. Prev Med 2010;51:509-516.
18. Lofters AK, Ng R, Lobb R. Primary care physician characteristics associated with cancer screening: A retrospective cohort study in Ontario, Canada. Cancer Med 2015;4:212-223.
19. Kiran T, Davie S, Singh D, et al. Cancer screening rates among transgender adults: Cross-sectional analysis of primary care data. Can Fam Physician 2019;65:e30-e37.
20. Cadman L, Waller J, Ashdown-Barr L, Szarewski A. Barriers to cervical screening in women who have experienced sexual abuse: An exploratory study. J Fam Plann Reprod Health Care 2012;38:214-220.
21. McGahan CE, Linn K, Guno P, et al. Cancer in First Nations people living in British Columbia, Canada: An analysis of incidence and survival from 1993 to 2010. Cancer Causes Control 2017;28:1105-1116.
22. Arbyn M, Smith SB, Temin S, et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: Updated meta-analyses. BMJ 2018;363:k4823.
23. Polman NJ, Ebisch RMF, Heideman DAM, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: A randomised, paired screen-positive, non-inferiority trial. Lancet Oncol 2019;20:229-238.
24. Nelson EJ, Maynard BR, Loux T, et al. The acceptability of self-sampled screening for HPV DNA: A systematic review and meta-analysis. Sex Transm Infect 2017;93:56-61.
25. Miller SM, Siejak KK, Schroeder CM, et al. Enhancing adherence following abnormal Pap smears among low-income minority women: A preventive telephone counseling strategy. J Natl Cancer Inst 1997;89:703-708.
26. Fish LJ, Moorman PG, Wordlaw-Stintson L, et al. Factors associated with adherence to follow-up colposcopy. Am J Health Educ 2013;44:293-298.
27. Stoler MH, Wright TC Jr, Sharma A, et al. High-risk human papillomavirus testing in women with ASC-US cytology: Results from the ATHENA HPV study. Am J Clin Pathol 2011;135:468-475.
Ms Gentile is the operations director for the Cervix Screening Program at BC Cancer. Ms Laurie W. Smith is the research program manager at Global Control of HPV Related Diseases and Cancer. Ms Brenda Smith is a manager of laboratory operations at BC Cancer. Dr Ogilvie is a professor in the School of Population and Public Health, Faculty of Medicine, University of British Columbia; Canada Research Chair in global control of HPV-related disease and cancer; the associate director at the Women’s Health Research Institute; and a senior public health scientist at the BC Centre for Disease Control. Dr Proctor is the medical director of the Cervix Screening Program at BC Cancer; a gynecologic oncologist at Vancouver General Hospital and BC Cancer; and a clinical assistant professor in the Faculty of Medicine, UBC.
Corresponding author: Ms Laura Gentile, laura.gentile@bccancer.bc.ca.