Original Research
Prevalence of intestinal parasites identified by microscopy prior to implementation of infectious diarrhea panel nucleic-acid amplification testing (IDP-NAAT): What are we missing?
ABSTRACT
Background: Since 2022, diagnostic laboratories in British Columbia have been advised to replace microscopy for intestinal ova and parasites with infectious diarrhea panel nucleic-acid amplification testing (IDP-NAAT). However, this multiplex assay captures only four common parasites: Cyclospora cayetanensis, Cryptosporidium spp., Entamoeba histolytica, and Giardia spp.
Methods: An audit was conducted from September 2022 to August 2023, 1 year prior to implementation of IDP-NAAT in LifeLabs BC, when microscopy was the method used to identify intestinal parasites.
Results: Pathogenic parasites were identified in 6149 of 52 221 stool specimens. The most common pathogens were Blastocystis hominis (78.47%), Dientamoeba fragilis (12.23%), Giardia spp. (4.93%), and Cyclospora spp. (1.07%). Entamoeba histolytica/dispar, Strongyloides stercoralis, Cryptosporidium spp., Ascaris lumbricoides, Diphyllobothrium spp., Enterobius vermicularis, Hymenolepis nana, Schistosoma mansoni, Taenia spp., Trichuris trichiura, and Clonorchis sinensis each accounted for less than 1% of the pathogenic parasites identified. Enterobius vermicularis was also identified in 46 of 1569 pinworm paddle specimens.
Conclusions: Several potentially pathogenic parasites could be missed if only IDP-NAAT is used to detect intestinal parasites. If indicated, microscopy orders would be needed to capture parasites not detected by IDP-NAAT.
Many parasitic pathogens could be missed if only IDP-NAAT is used to diagnose intestinal parasitic infections.
Background
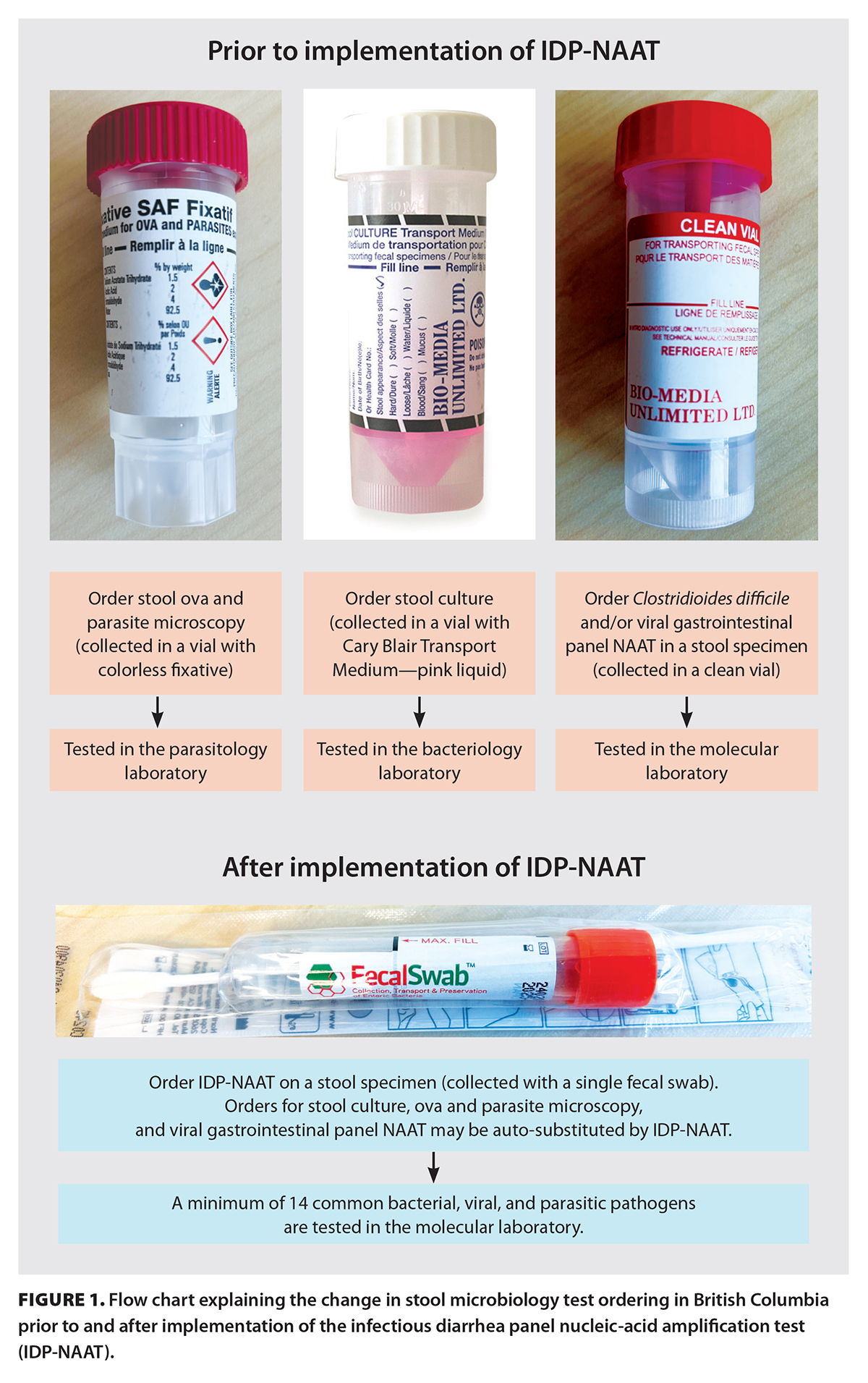 Traditionally, stool culture and microscopy for ova and parasites are the diagnostic methods of choice to detect intestinal pathogens. In 2022, diagnostic laboratories in British Columbia were advised to replace this testing method with the infectious diarrhea panel nucleic-acid amplification test (IDP-NAAT).[1] It combines a multiple gene target (multiplex) that detects a minimum of 14 common viral, bacterial, and parasitic pathogens [Box 1; Figure 1]. The BC Guidelines state that the list of pathogens may be modified periodically, in line with changes in epidemiology and technology. Potential pathogens to be considered include parasites such as Blastocystis hominis and Dientamoeba fragilis, even though they may not be pathogenic in each case.[2] The minimum pathogen list in the BC Guidelines includes only four parasites, while the number of possible intestinal parasites can be countless.[2] Moreover, the BC Guidelines recommend that if either stool culture or microscopy for ova and parasites is ordered, the laboratories will automatically substitute with IDP-NAAT and, thus, potentially miss the correct diagnosis. The BC Guidelines state that stool microscopies may be warranted if patients have a history of recent travel or immigration from low- or middle-income countries or are severely immunocompromised.
Traditionally, stool culture and microscopy for ova and parasites are the diagnostic methods of choice to detect intestinal pathogens. In 2022, diagnostic laboratories in British Columbia were advised to replace this testing method with the infectious diarrhea panel nucleic-acid amplification test (IDP-NAAT).[1] It combines a multiple gene target (multiplex) that detects a minimum of 14 common viral, bacterial, and parasitic pathogens [Box 1; Figure 1]. The BC Guidelines state that the list of pathogens may be modified periodically, in line with changes in epidemiology and technology. Potential pathogens to be considered include parasites such as Blastocystis hominis and Dientamoeba fragilis, even though they may not be pathogenic in each case.[2] The minimum pathogen list in the BC Guidelines includes only four parasites, while the number of possible intestinal parasites can be countless.[2] Moreover, the BC Guidelines recommend that if either stool culture or microscopy for ova and parasites is ordered, the laboratories will automatically substitute with IDP-NAAT and, thus, potentially miss the correct diagnosis. The BC Guidelines state that stool microscopies may be warranted if patients have a history of recent travel or immigration from low- or middle-income countries or are severely immunocompromised.
LifeLabs BC implemented IDP-NAAT in September 2023. Our laboratory conducted a retrospective audit on all intestinal parasites detected in our regional microbiology laboratories from 1 September 2022 to 31 August 2023, 1 year prior to implementation of IDP-NAAT. Our regional microbiology laboratories are connected with 129 collection centres in urban and rural communities in the province, and they provided the laboratory data on intestinal parasites, which indicated their local prevalence in community settings. The aim of this study was to identify intestinal parasites that would not be detected by IDP-NAAT and postulate plans to ensure the correct diagnosis is not missed.
Methods
Microscopies for ova and parasites in stool specimens
Microscopies for ova and parasites in stool specimens were ordered by clinicians. Patients or their caregivers were instructed to provide stool specimens in a clean vial with no liquid medium and a vial with sodium acetate–acetic acid–formalin fixative, which were then transported to the regional microbiology laboratories. Using a disposable plastic pipette, trained medical laboratory technologists placed a small amount of well-mixed sediment of the stool specimen on a plain glass microscope slide, which was then pressed by a coverslip. The technologists examined the entire area of the coverslip, first under 100× magnification and then under 400× magnification if suspicious features were seen. Iodine or carbol fuschin stain was used to enhance the detection of oocysts. When the presence of Cryptosporidium spp. or Cyclospora spp. was suspected, acid-fast stain was used. When testing for Strongyloides spp. or Schistosoma spp. was requested, a minimum of three slides were examined. The technologists were instructed to read each concentrate for an average of 9 minutes. The presence of ova, cysts, trophozoites, oocysts, larvae, adult worms of pathogenic parasites, and nonpathogenic parasites was reported.
Microscopies for pinworm paddle specimens
Microscopies for pinworm paddle specimens were ordered by clinicians. Patients or their caregivers were instructed to press the sticky surface of the pinworm paddle against the anal area early in the morning before arising or before bowel movement. The paddles were put in a vial and then transported to the regional microbiology laboratories. Each specimen was placed sticky side up on a glass microscope slide. Under 100× magnification, the technologists systematically examined the entire area of the paddle. The presence of pinworm (Enterobius vermicularis) ova and parasites was reported.
Data collection and analysis
Microscopies for ova and parasites and pinworm paddle orders were conducted from 1 September 2022 to 31 August 2023. The Microbiology Electronic Worksheet System software (version 5.00.267; LifeLabs, Toronto, ON) was used to generate data from all the microscopies. An entire year of data from patients of all ages and sexes was collected to reduce bias due to seasonality, differences in clinical practices, and other potential confounders. GraphPad Prism software (version 6.0c; GraphPad Software Incorporated, Boston, MA) was used to perform statistical analysis when needed.
Results
Microscopies for ova and parasites in stool specimens
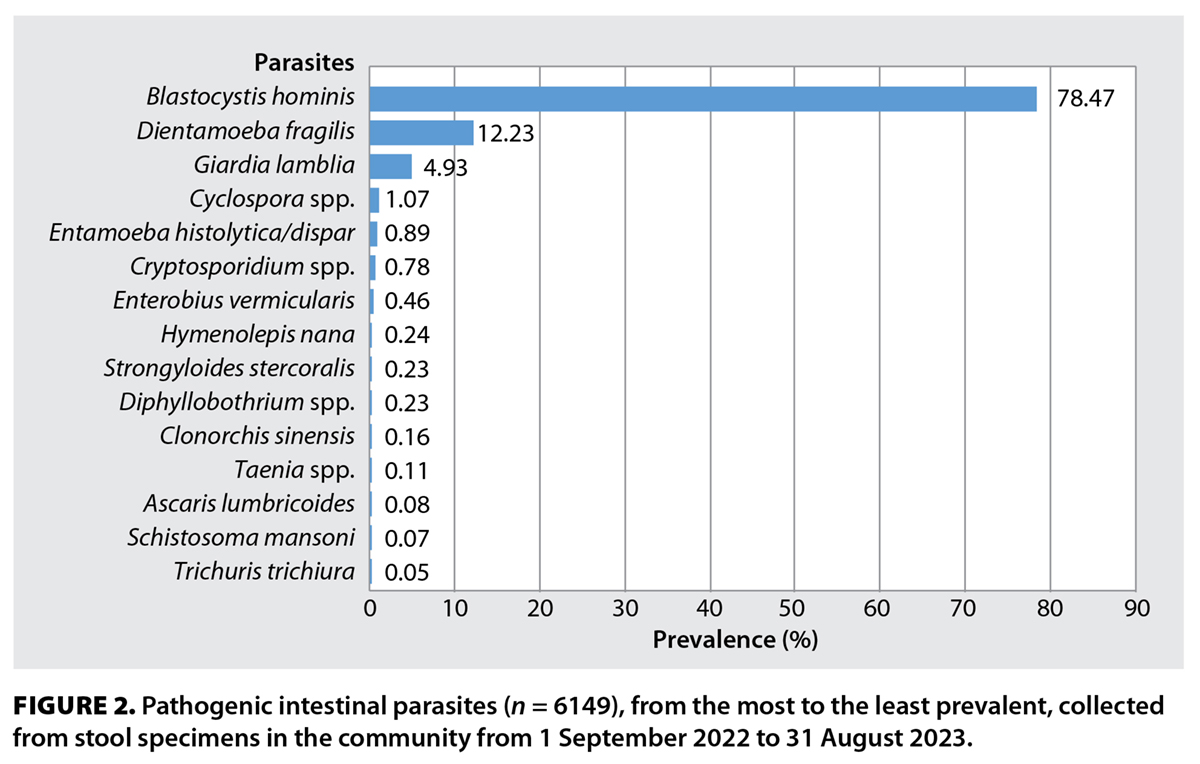 Pathogenic and nonpathogenic parasites were identified in 6149 and 1016 of the 52 221 stool specimens, respectively. The most common pathogens were Blastocystis hominis (78.47%), Dientamoeba fragilis (12.23%), Giardia lamblia (4.93%), and Cyclospora spp. (1.07%). Entamoeba histolytica/dispar, Cryptosporidium spp., Enterobius vermicularis, Hymenolepis nana, Strongyloides stercoralis, Diphyllobothrium spp., Clonorchis sinensis, Taenia spp., Ascaris lumbricoides, Schistosoma mansoni, and Trichuris trichiura each accounted for less than 1% of pathogenic parasites identified [Figure 2].
Pathogenic and nonpathogenic parasites were identified in 6149 and 1016 of the 52 221 stool specimens, respectively. The most common pathogens were Blastocystis hominis (78.47%), Dientamoeba fragilis (12.23%), Giardia lamblia (4.93%), and Cyclospora spp. (1.07%). Entamoeba histolytica/dispar, Cryptosporidium spp., Enterobius vermicularis, Hymenolepis nana, Strongyloides stercoralis, Diphyllobothrium spp., Clonorchis sinensis, Taenia spp., Ascaris lumbricoides, Schistosoma mansoni, and Trichuris trichiura each accounted for less than 1% of pathogenic parasites identified [Figure 2].
Microscopies for pinworm paddle specimens
Enterobius vermicularis was identified in 46 of 1569 pinworm paddle specimens.
Discussion
The results of this study suggest that several potentially pathogenic parasites could be missed if only IDP-NAAT is used to detect intestinal parasites. The most common intestinal parasitic pathogens identified were Blastocystis hominis (78.47%) and Dientamoeba fragilis (12.23%); however, it remained controversial whether they were true pathogens in each clinical case. In the absence of other diagnoses, it may be important to report when patients’ symptoms clinically correlate with the presence of these parasites, which can be roughly quantified (e.g., rare, few, many) in order to help clinicians determine the significance of their symptoms.[2] The current IDP-NAAT would not be able to report the presence of these parasites or quantify the amount of any parasite in a test sample.
Most of the parasites detected in this study accounted for less than 1% of all those found. Although it can be argued that the incidences of parasites missed by IDP-NAAT were statistically insignificant, statistics do not always apply in incidents of patient safety: one severe, highly nonconforming event, regardless of probability, would be considered significant.[3] If stool microscopy orders were automatically replaced with IDP-NAAT, as the BC Guidelines suggest, many intestinal parasite diagnoses could be missed. Clinicians do not always order intestinal parasite testing simply to determine the cause of diarrhea. They may look for the following parasites in other clinical situations:
- Ascaris lumbricoides—associated with eosinophilia, intestinal blockage, and impaired growth.[4,5]
- Clonorchis sinensis—associated with eosinophilia, gallbladder obstruction, jaundice, and hepatomegaly.[6]
- Diphyllobothrium spp.—associated with weight loss, vitamin B12 deficiency, pernicious anemia, intestinal obstruction, and gallbladder disease.[7]
- Enterobius vermicularis—associated with perianal itching.[8]
- Hookworm—associated with anemia and chronic protein deficiency, especially in children.[4]
- Schistosoma spp.—associated with fever and hematochezia (even though serologic testing may be preferred).[9,10]
- Strongyloides stercoralis—associated with hyperinfection syndrome, characterized by abdominal pain, diffuse pulmonary infiltrates, and septicemia or meningitis (even though serologic testing may be preferred).[11,12]
- Taenia solium—associated with cysticercosis (even though neuroimaging and serologic testing may be preferred).[13]
- Trichuris trichiura—associated with anemia and rectal prolapse.[4]
Implications for clinicians
 Although IDP-NAAT, in general, is more sensitive than microscopy and culture testing, a “multiplex” IDP-NAAT is not “omniplex.” Now that BC diagnostic laboratories are transitioning to IDP-NAAT, which captures 14 common intestinal pathogens, it is expected that clinicians will need to be more familiar with the various pathogens beyond these 14 common ones to aid their differential diagnoses. If the multiplex IDP-NAAT does not include targets for the potential pathogens in their differentials, additional specific testing would be required, especially if the patient is still symptomatic and has no clear diagnosis.
Although IDP-NAAT, in general, is more sensitive than microscopy and culture testing, a “multiplex” IDP-NAAT is not “omniplex.” Now that BC diagnostic laboratories are transitioning to IDP-NAAT, which captures 14 common intestinal pathogens, it is expected that clinicians will need to be more familiar with the various pathogens beyond these 14 common ones to aid their differential diagnoses. If the multiplex IDP-NAAT does not include targets for the potential pathogens in their differentials, additional specific testing would be required, especially if the patient is still symptomatic and has no clear diagnosis.
Another potential change of practice is the timing to order test of cure for occupational clearance, especially for bacterial intestinal infections, depending on local occupational health and public health policies. IDP-NAAT is highly sensitive and could generate reactive results, even when patients are no longer infectious.[14] The BC Guidelines do not specify whether a test of cure is needed for each pathogen. Laboratories may have their own laboratory-developed assays and therefore no published guidance on when to order repeat testing, if indicated.
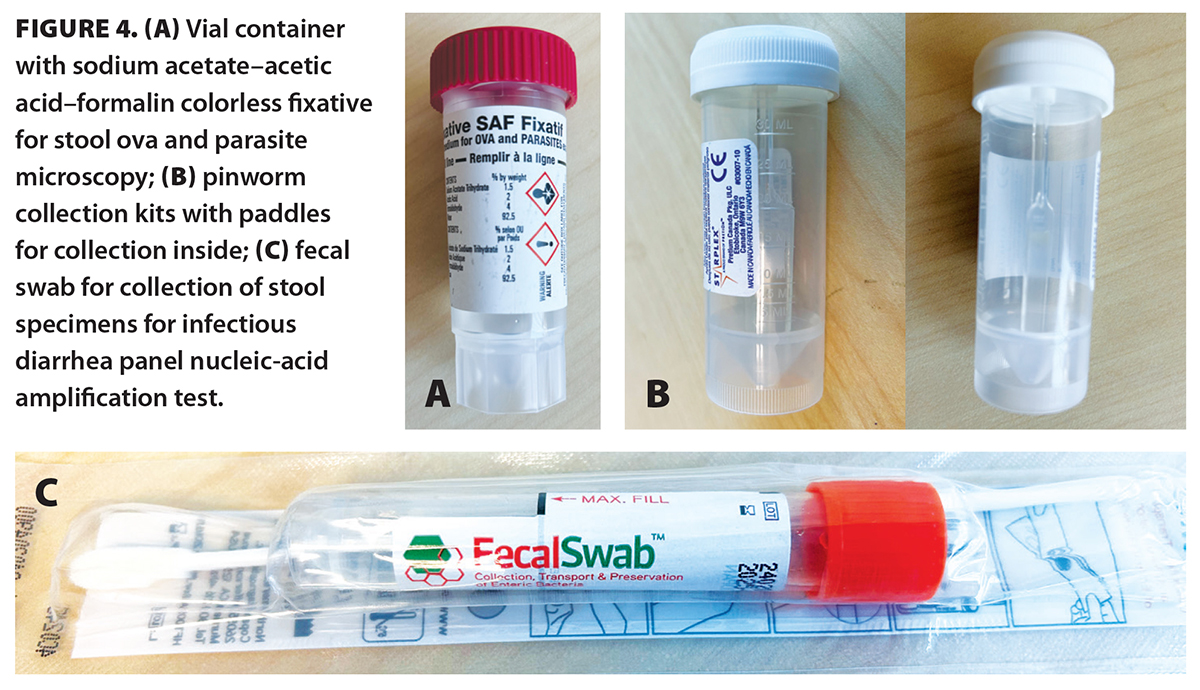 Furthermore, if laboratories are transitioning to automatic substitution of stool microscopy orders with IDP-NAAT, clinicians would have to clearly indicate on their order requisition forms that microscopy is needed and provide the rationale for it. An example of how to fill out a requisition form is provided in Figure 3. To further prevent errors, when handing requisition forms to patients, clinicians may want to remind them that they should anticipate receiving vials (for stool microscopy) rather than swabs (IDP-NAAT) from the laboratory patient service centres [Figure 4]. These tips are summarized in Box 2.
Furthermore, if laboratories are transitioning to automatic substitution of stool microscopy orders with IDP-NAAT, clinicians would have to clearly indicate on their order requisition forms that microscopy is needed and provide the rationale for it. An example of how to fill out a requisition form is provided in Figure 3. To further prevent errors, when handing requisition forms to patients, clinicians may want to remind them that they should anticipate receiving vials (for stool microscopy) rather than swabs (IDP-NAAT) from the laboratory patient service centres [Figure 4]. These tips are summarized in Box 2.
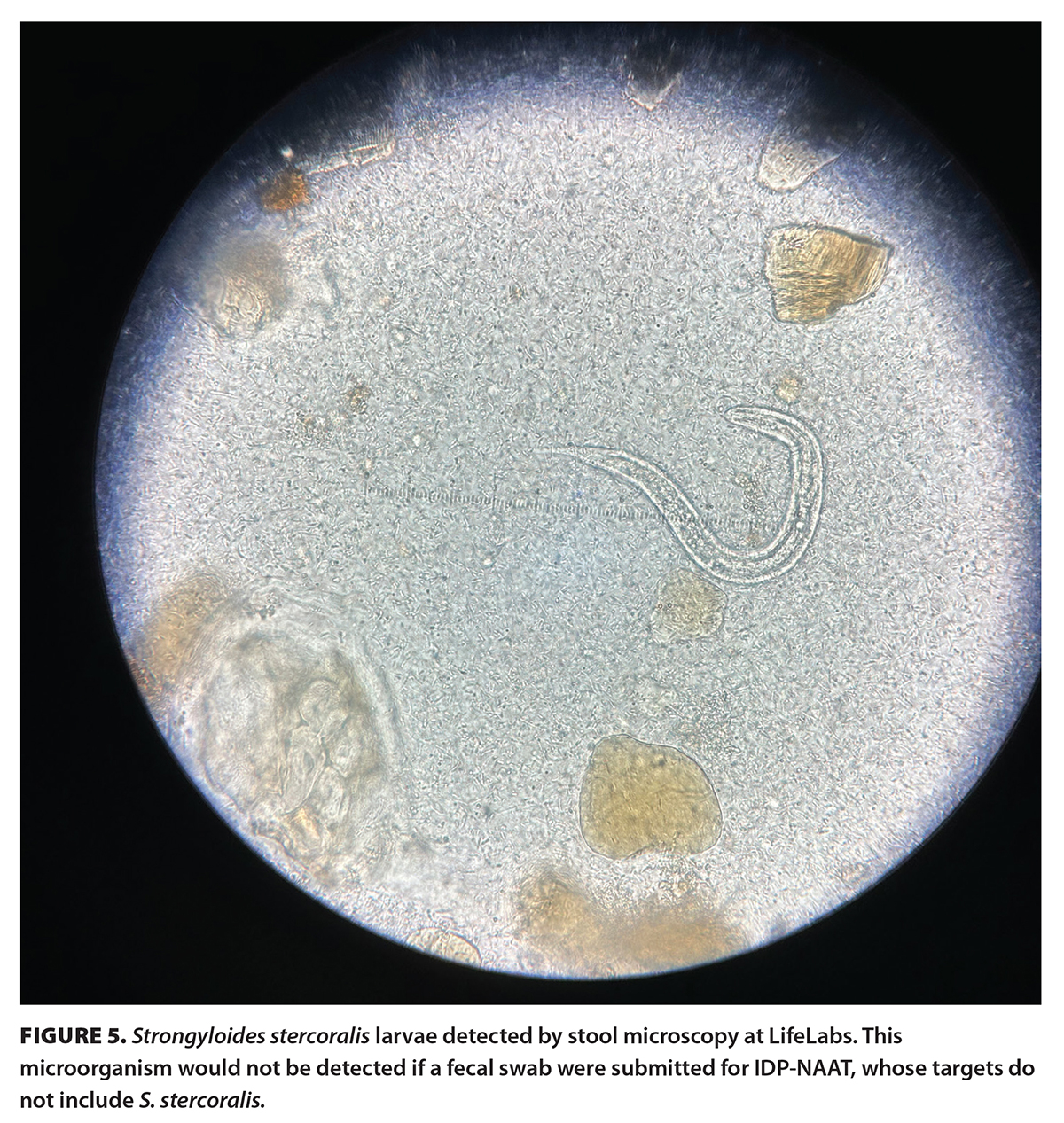 Although the BC Guidelines recommend stool microscopies if patients have a history of recent travel or immigration from low- or middle-income countries or are severely immunocompromised, these criteria may not capture all at-risk patients. For instance, Diphyllobothrium infections generally occur in the northern hemisphere, including Europe; newly independent states of the former Soviet Union; North America; and Asia.[7] Fish-borne parasitic infections, secondary to Anisakis spp. and Diphyllobothrium spp., are endemic in cosmopolitan regions in Japan.[15] Several intestinal parasites, including Ascaris lumbricoides, Trichuris trichiura, and Taenia spp., isolated from human stool specimens in Ontario were deemed to be endemic in Canada rather than imported.[16] Strongyloidiasis could be asymptomatic or cause minimal symptoms in its acute phase [Figure 5]. Thus, it is not apparent in recent travelers and immigrants, but clinical disease can be lifelong and turn into hyperinfection or disseminated disease when patients become immunocompromised.[11,12]
Although the BC Guidelines recommend stool microscopies if patients have a history of recent travel or immigration from low- or middle-income countries or are severely immunocompromised, these criteria may not capture all at-risk patients. For instance, Diphyllobothrium infections generally occur in the northern hemisphere, including Europe; newly independent states of the former Soviet Union; North America; and Asia.[7] Fish-borne parasitic infections, secondary to Anisakis spp. and Diphyllobothrium spp., are endemic in cosmopolitan regions in Japan.[15] Several intestinal parasites, including Ascaris lumbricoides, Trichuris trichiura, and Taenia spp., isolated from human stool specimens in Ontario were deemed to be endemic in Canada rather than imported.[16] Strongyloidiasis could be asymptomatic or cause minimal symptoms in its acute phase [Figure 5]. Thus, it is not apparent in recent travelers and immigrants, but clinical disease can be lifelong and turn into hyperinfection or disseminated disease when patients become immunocompromised.[11,12]
Implications for epidemiologists
Changes in test methods can lead to pseudo-outbreak.[17] An increase in incidents of certain pathogens could be due to the superior sensitivity of IDP-NAAT compared with microscopy.[18] Epidemiologists may need to determine the implementation dates of IDP-NAAT in different laboratories and set new baseline surveillance rates. The prevalence of some intestinal pathogens, such as Blastocystis hominis and Dientamoeba fragilis, may seem to decline, but it may be that they are not being identified by IDP-NAAT.
Implications for laboratorians
Similar to epidemiologists, laboratorians should conduct their own surveillance studies of intestinal pathogens detected in their laboratories. If there has been a significant decrease in the prevalence of intestinal pathogens since the implementation of IDP-NAAT, it could be that they are not being identified by that test. Consideration can be given to incorporating those pathogens into the multiplex panel.
Comparison to other studies
In this study, the most common intestinal parasitic pathogens identified were Blastocystis hominis and Dientamoeba fragilis. They were also the most predominant intestinal parasites found in stool testing among refugees at a primary care clinic in Toronto, Ontario.[19] Similar to our study, that study found infrequent occurrences of parasitic helminths,[19] which are not among the 14 common intestinal pathogens listed in the BC Guidelines. The variety of parasites identified in our study was consistent with studies of parasitic diseases that were conducted in Canada in the 1970s and 2000s.[16,20] However, unlike those studies, our study did not detect Toxoplasma spp. or Trichinella spiralis, which are diagnosed mainly using serological testing.
Study limitations
A major limitation of this study was the exclusion of data from hospitals, whose clinicians may have different indications warranting the need for stool microscopies in addition to IDP-NAAT. Hospital laboratories are welcome to conduct their own audits to observe changes in pathogen prevalence due to implementation of IDP-NAAT. Another limitation of this study was that not all of the 53 790 orders (52 221 stool and 1569 pinworm paddle specimens) were evaluated to determine whether a diagnosis could be missed. This study was meant to hypothesize about diagnoses that could be missed if only IDP-NAAT was used for diagnosing intestinal parasitic infections. In addition, this study did not investigate bacterial pathogens that could be missed, because IDP-NAAT recommends testing only seven common bacterial pathogens but not some rare ones such as Aeromonas spp., Plesiomonas spp., Edwardsiella spp., and Yersinia spp., other than Yersinia enterocolitica.[1,21,22] Further studies may determine whether testing for these pathogens should be included in IDP-NAAT. Despite this study’s limitations, its major strength was the inclusion of almost all community microbiology data; therefore, the results should be generalizable to the community population in BC.
Conclusions
This 1-year study demonstrated that many parasitic pathogens could be missed if only IDP-NAAT is used to diagnose intestinal parasitic infections. If indicated, microscopy orders would be needed to capture these additional parasites.
Acknowledgments
The author would like to thank all the LifeLabs microbiology staff, including microbiologists, medical scientists, medical laboratory technologists, technicians and assistants, and information technology personnel, for their technical support in the study.
Funding
None.
Competing interests
Dr Yeung has been paid for working as a microbiologist, physician, and pharmacist. The views and opinions expressed are those of the author and do not necessarily reflect the views or positions of his employers.
BOX 1. The minimum 14 pathogens in the infectious diarrhea panel nucleic-acid amplification test used in each diagnostic laboratory in BC.
- Viral pathogen: adenovirus 40/41, norovirus GI/GII, rotavirus.
- Bacterial pathogen: Campylobacter spp., Clostridioides difficile, Shiga toxin-producing Escherichia coli, Salmonella spp., Shigella spp., Yersinia enterocolitica, Vibrio spp.
- Parasitic pathogens: Cyclospora cayetanensis, Cryptosporidium spp., Entamoeba histolytica, Giardia spp.
BOX 2. Practice tips on how to prevent auto-substitution of microscopy with infectious diarrhea panel orders, if clinically indicated.
When and how to order microscopy rather than infectious diarrhea panel (IDP)—use the acronym PICH:
- Consider stool microscopy if parasitic infections are in your differential diagnoses but are beyond the four parasites in the IDP (i.e., Cyclospora cayetanensis, Cryptosporidium spp., Entamoeba histolytica, Giardia spp.). Consider pinworm collection kit when pinworm (Enterobius vermicularis) is suspected.
- Indicate the rationale for why stool microscopy is needed in the “Diagnosis and indications” section of the laboratory requisition form (e.g., recent travel or immigration from a low- or middle-income country, immunocompromised).
- Clarify in the “Other tests” box of the laboratory requisition form that microscopy, not IDP, is needed.
- When handing out the requisition form, remind the patient that a vial container, not a swab, should be given.
This article has been peer reviewed.
 |
| This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. |
References
1. Government of British Columbia. Infectious diarrhea—Guideline for investigation. Last updated 30 November 2023. Accessed 13 March 2024. www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/bc-guidelines/infectious-diarrhea-guideline-for-investigation.
2. Miller JM, Binnicker MJ, Campbell S, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis 2018;67:e1-e94.
3. Clinical and Laboratory Standard Institute. CLSI QMS11: Nonconforming event management. 2nd ed. 2015. Accessed 14 March 2024. https://clsi.org/standards/products/quality-management-systems/documents/qms11/.
4. Kamb M, Roy S. Helminths, soil-transmitted. In: CDC Yellow Book 2024: Travel-associated infections and diseases. Centers for Disease Control and Prevention. Accessed 13 March 2024. wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/helminths-soil-transmitted.
5. Centers for Disease Control and Prevention. About Ascariasis. Accessed 21 March 2024. www.cdc.gov/sth/about/ascariasis.html.
6. Roy S, Cantey P. Flukes, liver. In: CDC Yellow Book 2024: Travel-associated infections and diseases. Centers for Disease Control and Prevention. Accessed 21 March 2024. wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/flukes-liver.
7. Centers for Disease Control and Prevention. About Diphyllobothrium. Accessed 21 March 2024. www.cdc.gov/diphyllobothrium/about.
8. Chancey R, Kamb M. Enterobiasis/pinworm. In: CDC Yellow Book 2024: Travel-associated infections and diseases. Centers for Disease Control and Prevention. Accessed 13 March 2024. wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/enterobiasis-pinworm.
9. Montgomery S, Secor WE. Schistosomiasis. In: CDC Yellow Book 2024: Travel-associated infections and diseases. Centers for Disease Control and Prevention. Accessed 13 March 2024. wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/schistosomiasis.
10. Boggild A, Ghesquiere W, McCarthy A. Fever in the returning international traveller initial assessment guidelines: Committee to Advise on Tropical Medicine and Travel (CATMAT). Can Commun Dis Rep 2011;37:1-15.
11. Montgomery S, Chancey R, Kamb M. Strongyloidiasis. In: CDC Yellow Book 2024: Travel-associated infections and diseases. Centers for Disease Control and Prevention. Accessed 2024 Mar 13. wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/strongyloidiasis.
12. Boggild AK, Libman M, Greenaway C, McCarthy AE. CATMAT statement on disseminated strongyloidiasis: Prevention, assessment and management guidelines. Can Commun Dis Rep. 2016;42:12-19.
13. Cantey P, Roy S. Cysticercosis. In: CDC Yellow Book 2024: Travel-associated infections and diseases. Centers for Disease Control and Prevention. Accessed 21 March 2024. wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/cysticercosis.
14. Park S, Hitchcock MM, Gomez CA, Banaei N. Is follow-up testing with the FilmArray gastrointestinal multiplex PCR panel necessary? J Clin Microbiol 2017;55:1154-1161.
15. Nawa Y, Hatz C, Blum J. Sushi delights and parasites: The risk of fishborne and foodborne parasitic zoonoses in Asia. Clin Infect Dis 2005;41:1297-1303.
16. Lee MB. Everyday and exotic foodborne parasites. Can J Infect Dis 2000;11:155-158.
17. Curran ET. Outbreak Column 7: Pseudo-outbreaks (part 1). J Infect Prev 2013;14:69-74.
18. Stensvold CR, Nielsen HV. Comparison of microscopy and PCR for detection of intestinal parasites in Danish patients supports an incentive for molecular screening platforms. J Clin Microbiol 2012;50:540-541.
19. Müller F, Chandra S, Bogoch II, et al. Intestinal parasites in stool testing among refugees at a primary care clinic in Toronto, Canada. BMC Infect Dis 2022;22:249.
20. Croll NA, Gyorkos TW. Parasitic disease in humans: The extent in Canada. Can Med Assoc J 1979;120:310-312.
21. Loftus CG, Harewood GC, Cockerill FR 3rd, Murray JA. Clinical features of patients with novel Yersinia species. Dig Dis Sci 2002;47:2805-2810.
22. Yeung EYH. A case series of diarrheal diseases associated with Yersinia frederiksenii. Infect Dis Rep 2021;13:552-557.
Dr Yeung is a clinical assistant professor in the Faculty of Medicine and the Faculty of Pharmaceutical Sciences at the University of British Columbia and a staff medical microbiologist at LifeLabs BC.
