Original Research
Prescription factors contributing to new long-term opioid use in British Columbia between 2013 and 2017
ABSTRACT
Background: The opioid epidemic has been linked to liberal opioid prescribing practices of physicians. We re-examined trends in opioid prescription practices in British Columbia that have led to new long-term use.
Methods: A data set of community-dispensed opioids from January 2013 to December 2017 was used to identify opioid-naive individuals. Opioid prescriptions were analyzed to describe new long-term and non-long-term users. Initial prescription factors associated with longer use were estimated.
Results: In total, 19 785 practitioners issued 15 693 867 opioid prescriptions to 1 692 035 patients; 7.2% of opioid-naive individuals became new long-term users. Compared with non-long-term users, new long-term users were first prescribed a total opioid dose 1.7 times higher, and most received their prescription from a family physician. By the end of the study, 59.8% of new long-term users had stopped opioid use, 37.9% continued use, and 2.3% transitioned to methadone/buprenorphine. Longer duration of opioid use was associated with older age, a first prescription of fentanyl or butorphanol, and a first prescriber specialty type of psychiatry.
Conclusions: Limitations included the fact that first prescriptions may have been renewals of hospital prescriptions and indications were unknown. This study may inform prescribers how opioid prescriptions impact long-term use.
Changing opioid prescription practices is key to reducing opioid overdose.
 Background
Background
Long-term opioid therapy is associated with significant side effect profiles.[1-4] The prevalence of long-term opioid therapy has been reported to be 5.4% in the United States and 17.6% in Canada.[5,6] Within most populations and health care settings, the rising prevalence of long-term opioid therapy has been associated with a corresponding increase in opioid misuse and opioid-related overdose and mortality.[2,3,5-7] The prevalence of opioid misuse (i.e., the use of opioids differently from how they were prescribed) ranges between 21% and 29% in primary and tertiary care settings, respectively.[8] Compared with matched controls, long-term opioid therapy management of noncancer-related pain is associated with a 58% increased risk for all-cause mortality compared with non-opioid analgesia.[3] Since 2016, there has been an almost threefold increase in fatal overdose rates in British Columbia due to all illicit drugs, with 45 deaths per 100 000 individuals in 2023.[9] Notably, drug toxicity is now the leading cause of unnatural death in BC, surpassing deaths related to motor vehicle accidents.[10] Although these illicit drug deaths are not necessarily correlated with opioid prescriptions, it should be considered that 24% of all US opioid-related deaths in 2020 involved a prescription opioid.[11] There are several predictors of the use of long-term opioid therapy in opioid-naive patients, including patient characteristics (pain, medical conditions, and mental health conditions), sociodemographic influences, and prescription factors.[12]
The opioid epidemic has been linked to liberal opioid prescribing practices of physicians, increased availability of pharmaceutical opioids in the community, and increased opioid consumption.[13,14] Recognizing prescription opioids as an important contributor to the opioid epidemic is key to understanding and minimizing the potential harm when prescribing opioids.[12,15-17] This is especially relevant in the context of opioid-naive individuals. Some individuals may be only short-term users, while others become long-term users. In noncancer opioid-naive patients, the number of days supplied of the initial opioid prescription was the strongest predictor of long-term use.[12,16] Opioid type (long-acting or tramadol) and dose were also significant predictors of continued use.[12,16] A recent meta-analysis of observational studies on the use of prescribed opioids for chronic pain showed strong associations between overdose (fatal and nonfatal) and current substance use disorder; mental health diagnosis; pancreatitis; and prescription factors, such as multiple opioid prescribers, multiple dispensing pharmacies, prescription of morphine equivalents that were 90 mg or greater, and prescription of fentanyl.[18] Despite known risk factors of long-term opioid therapy, initial prescribing practices around the world vary substantially.[19,20] Jani and colleagues found that individuals in Taiwan were initially prescribed weak/moderate opioids at 8 morphine milligram equivalents (MME) per day, compared with individuals in the United Sates, who were initially prescribed higher-potency opioids (e.g., oxycodone, hydrocodone, hydromorphone) at nearly five times the MME (38 MME per day).[20] This variability in prescription practices in excess of clinical need increases the risk of opioid dependency, recreational use, opioid sharing/diversion, accidental overdose, and death.[21-23]
Within BC, there is a wide range of opioid prescription patterns.[24] A 5-year longitudinal population-based study by Yefet and colleagues found that 12% of individuals within the province were provided with an opioid prescription during the study period, and prescriptions varied within and across subspecialties by both specific opioid type and prescribed MME.[25] A better understanding of the relationship between initial prescriptions and new long-term use may thus inform and educate prescribers of opioids. Appropriate educational tools and resources for pain management may reduce the likelihood of patients developing problematic opioid use and the contribution of opioid prescriptions to the opioid epidemic. This study aims to build on the results of Yefet and colleagues[25] by describing trends in opioid prescriptions for new long-term users and exploring the prescription and prescriber factors associated with new long-term opioid use.
Methods
Ethics approval for this study was obtained from the University of British Columbia Children’s and Women’s Research Ethics Board (H18-01006). This study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments. This research was funded by the BC Children’s Hospital Research Institute’s Evidence to Innovation. This study conforms to the rules of data reporting outlined by Population Data BC.[26]
Data sources
This study was a secondary analysis of a de-identified, 5-year, population-based prescription data set obtained by Population Data BC that was used in a previous report that described opioid-prescribing patterns in BC.[25] The data set included all community-dispensed opioids in BC from 2013 to 2017. Each prescription included the drug identification number, date dispensed, quantity dispensed, days supplied, and patient age and sex. Request to analyze the indication for opioid prescriptions was denied due to patient confidentiality concerns.
Drug-specific details included chemical/generic name, drug strength, drug form units, and dosage form (e.g., two tablets). Oral MME was calculated for each drug using conversion factors from the US Centers for Disease Control’s 2017 oral MME guide.[27] Opioid formulations were grouped by specific opioid type. Tramadol and tapentadol were combined due to similar mechanisms of action.[28] Codeine and tramadol/tapentadol were characterized as weak opioids, and the remainder as strong opioids.
Prescriber specialty type, described in the data set analyzed by Yefet and colleagues,[25] was based on linked data obtained from the College of Physicians and Surgeons of BC.[29]
Inclusion/exclusion
Data cleaning was performed. Prescriptions within the first 6 months of the study period were excluded to meet the criteria of opioid-naive [Table 1].[30] Similarly, prescriptions associated with the start of an episode in the last 6 months of the study period were also excluded, because there was not enough follow-up time to define those episodes as potential long-term users (see episode duration definitions[30] in Table 1).
Study population
The study population was categorized into three user groups:
- Non-long-term users.
- New long-term users initially prescribed an opioid that was not methadone or buprenorphine.
- Long-term users initially prescribed methadone or buprenorphine.
Long-term users initially prescribed methadone or buprenorphine were separated from new long-term users because the latter were assumed very unlikely to be opioid-naive.
Statistical analysis
Descriptive data were used to summarize opioid user groups by patient factors (e.g., age, sex), prescription factors (e.g., MME, days supplied, prescriber specialty type, opioid type), and episode factors (e.g., type, duration, ongoing status at the end of the study period). For new long-term users, the prescriptions from incident long-term episodes with a daily MME greater than 90 MME per day were calculated. The number of specific opioid and prescriber specialty types per episode were summarized. Opioid use status at the end of the study period was determined.
The probability of continued opioid use by initial drug type prescribed was estimated using the Kaplan-Meier estimator. Those results were summarized graphically as the probability of continuing use at 1 year and 2 years after first prescription. The relationship between prespecified demographic and prescription factors and episode duration was analyzed using multivariable Cox proportional hazards models. For those models and Kaplan-Meier curves, we censored individuals at the end of their episode or the end of the study period, whichever came first. Results from Cox models were summarized as hazard ratios and 95% confidence intervals. All analyses were conducted using R statistical software, version 4.0.3.[31]
Results
 Study population
Study population
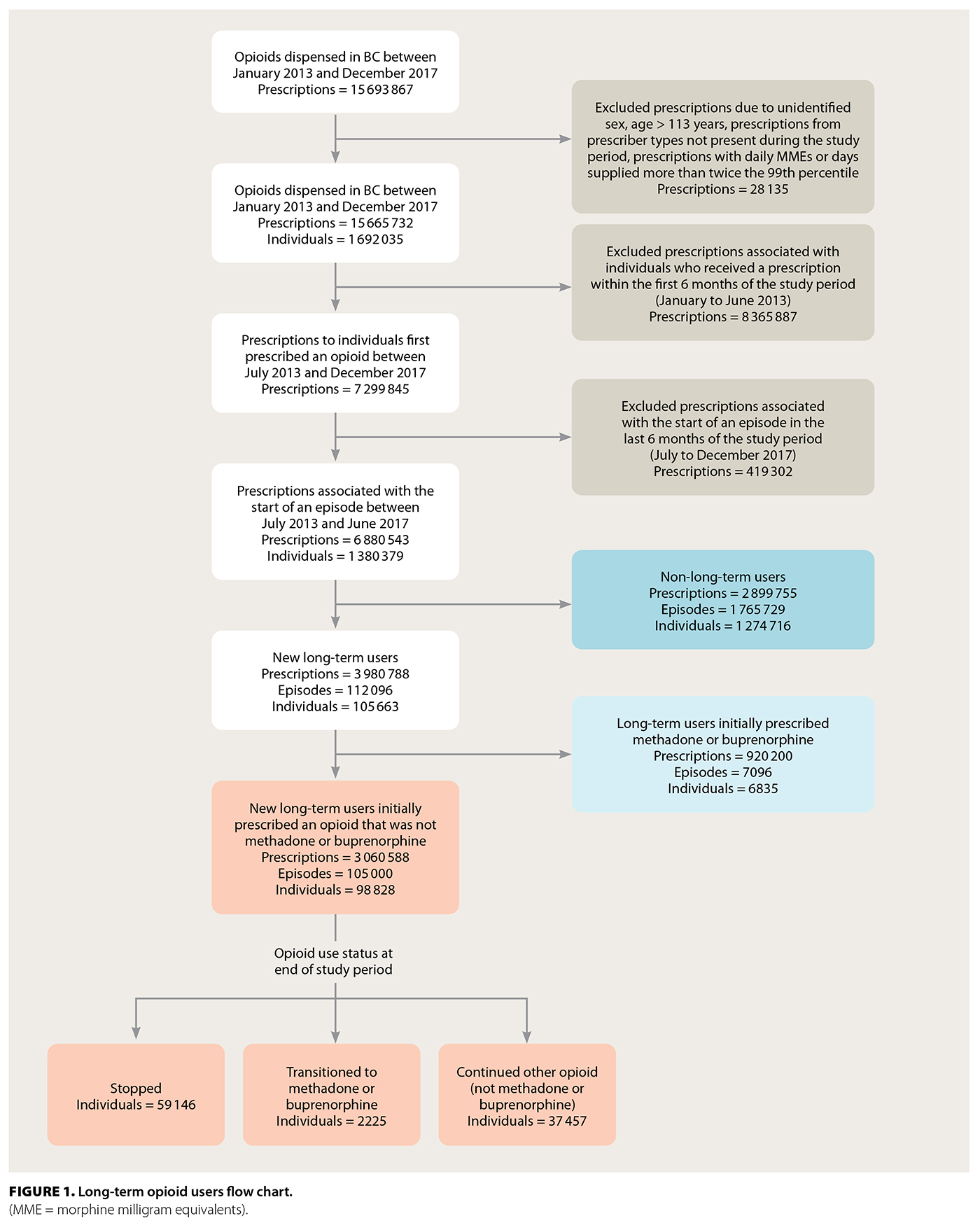
From January 2013 to December 2017, 15 693 867 opioid prescriptions were issued to 1 692 035 individuals in BC by 19 785 practitioners. During the last year of the study period, 3 223 935 opioid prescriptions were dispensed to 565 776 people within BC. This equates to 155 MME for every person in the province. These prescriptions were supplied to 11.5% of the BC population.
In total, 28 135 prescriptions were excluded from analysis due to unidentified sex, age greater than 113 years, inaccurate prescriber type, or prescriptions with daily MMEs or days supplied that were more than twice the 99th percentile. From the remaining 15 665 732 prescriptions, those in the first 6 months of the study (8 365 887) and those associated with an episode that began in the last 6 months of the study (419 302) were excluded. Of the included incident episodes, 42% of prescriptions were single or short-term use; 58% were long-term use [Figure 1].
Non-long-term users
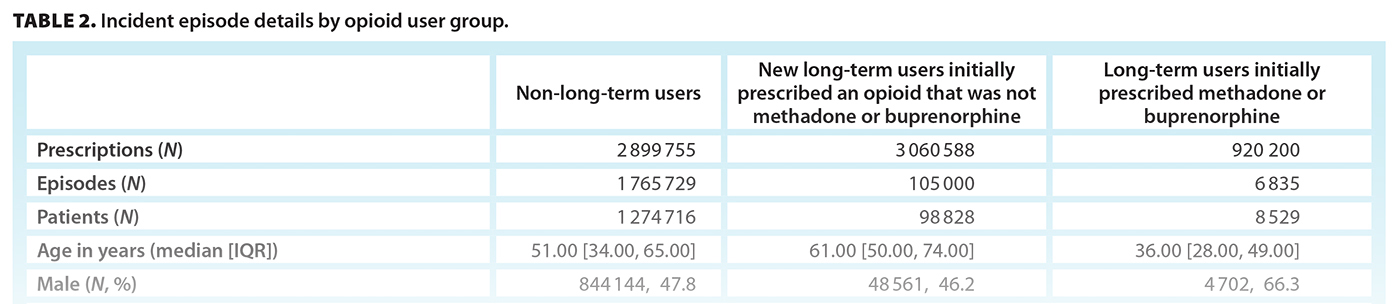
In total, 2 899 755 prescriptions were associated with 1 274 716 individuals with non-long-term-use (median age 51 years; 47.8% male) [Table 2]. The initial prescription median MME was 30.00 (IQR 18.75, 45.00), with a median day supply of 5.00 (IQR 3.00, 7.00) MME, giving a median total prescription (dose × days supplied) of 135.00 (IQR 90.00, 188.00) MME. Most initial prescriptions were for codeine (67.1%) or tramadol/tapentadol (18.8%). The most common prescriber specialties were family practice (48.7%), surgery (18.1%), and dentistry (15.6%). Most episode types were single prescription (71.1%), followed by short term (17.8%) and intermediate (11.1%).
 New long-term users
New long-term users
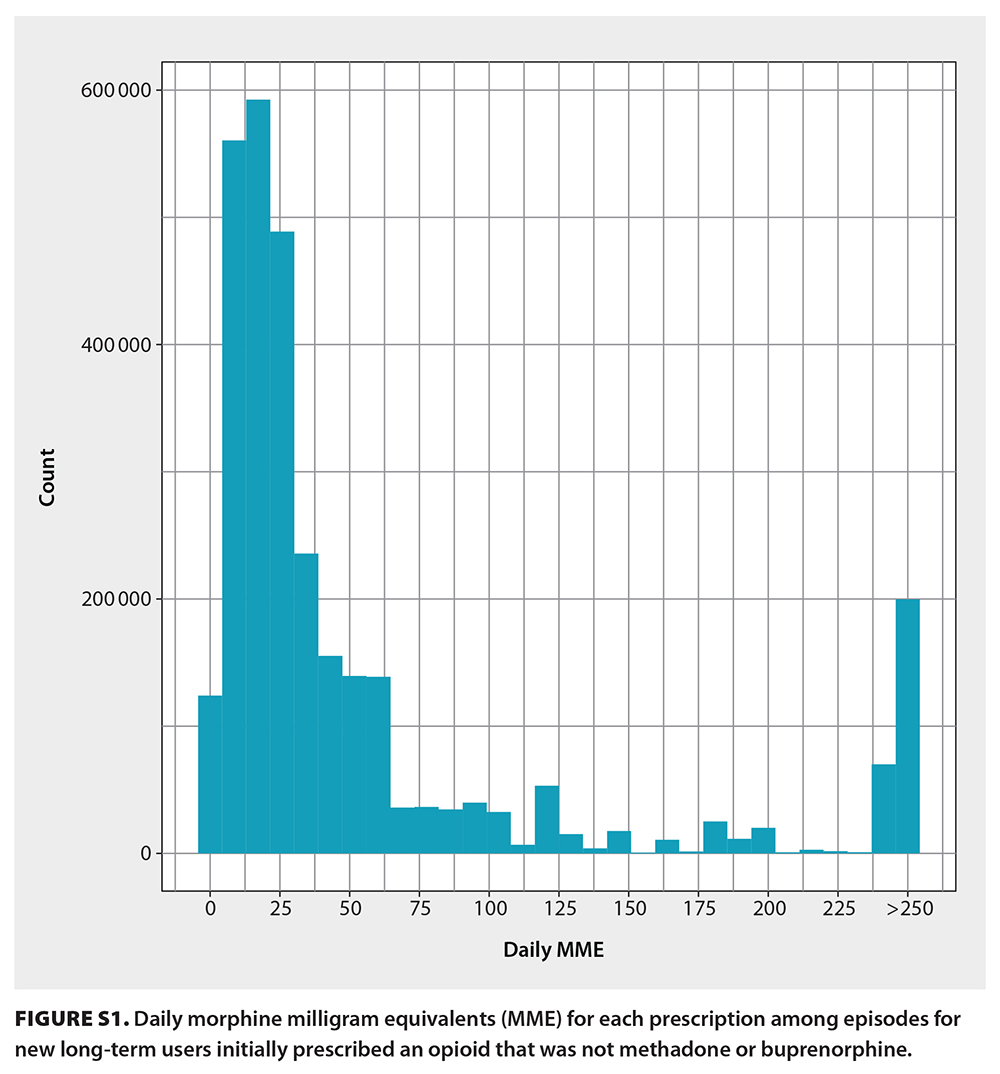
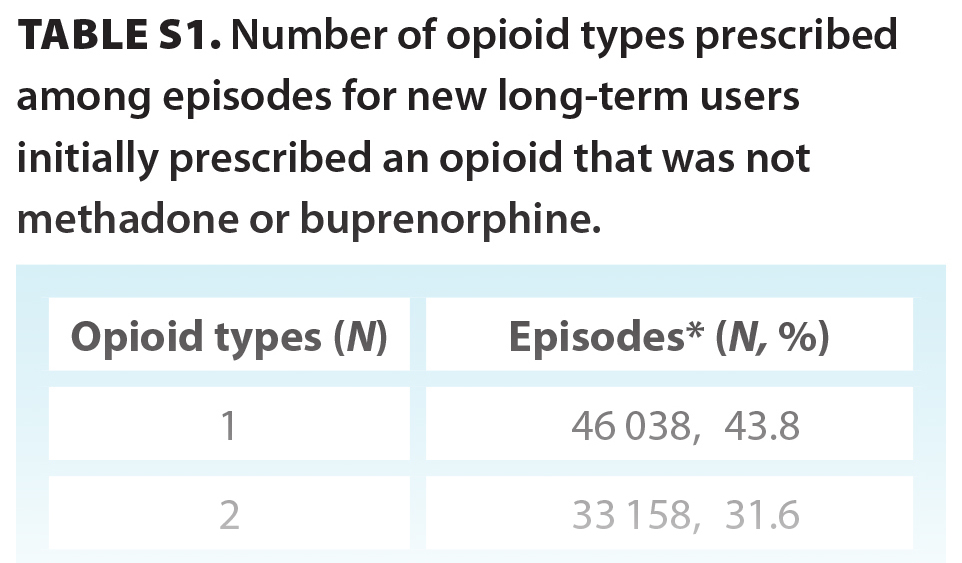
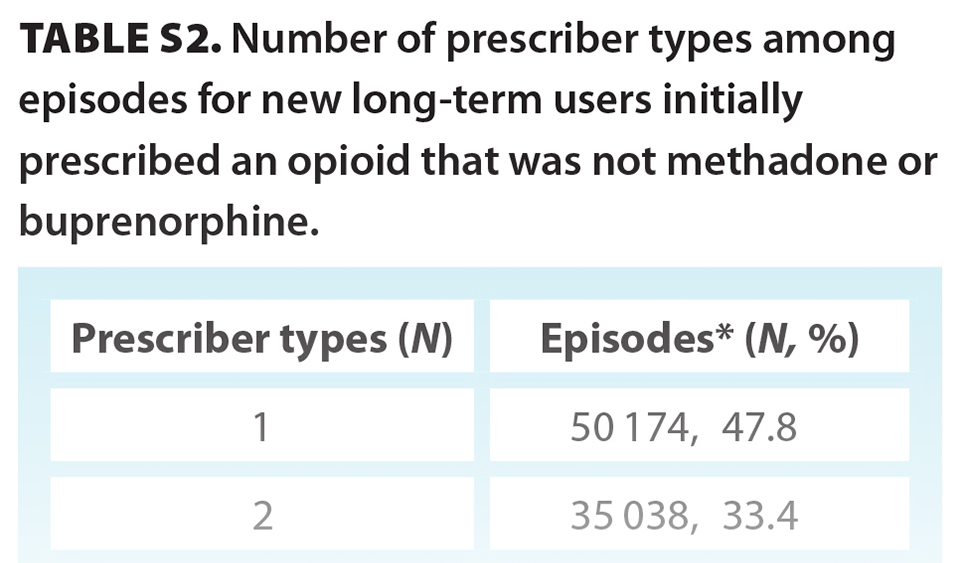
In total, 3 060 588 prescriptions were associated with 98 828 individuals who were new opioid-naive users who went on to have long-term episodes (median age 61 years; 46.2% male) [Table 2]. Overall, 7.2% of opioid-naive individuals became new long-term users during the study period. The initial prescription median MME was 22.50 (IQR 12.86, 36.00), with a median day supply of 10.00 (IQR 5.00, 25.00) MME, giving a median total prescription (dose × days supplied) of 225.00 (IQR 135.00, 405.00) MME. In total, 515 126 (16.8%) prescriptions from incident long-term episodes had a daily MME greater than 90 [Figure S1]. Most initial prescriptions were codeine (53.7%), tramadol/tapentadol (19.9%), or hydromorphone (13.4%) [Table 2]. Approximately one-quarter (24.2%) of all long-term episodes involved prescriptions for three or more opioid types over the duration of an episode [Table S1]. The most common prescriber specialty was family practice (79.5%); however, 18.8% of episodes involved prescriptions from three or more prescriber specialty types [Table S2]. At the end of the study period, individuals had one of three outcomes: 59.8% (59 146) stopped opioid use, 37.9% (37 457) continued opioid use, and 2.3% (2 225) transitioned to methadone or buprenorphine [Figure 1]. Of the 59 146 individuals who stopped opioid use, 3445 had received a methadone or buprenorphine prescription prior to stopping.
 |
 |
Long-term users
In total, 920 200 prescriptions were associated with 6835 individuals who were long-term users that were first prescribed methadone or buprenorphine (median age 36 years; 66.3% male) [Table 2]. The initial prescription median MME of methadone or buprenorphine was 120.00 (IQR 42.00, 240.00), with a median day supply of 2 days, giving a total prescription of 225.00 (IQR 135.00, 450.00) MME. The initial prescription was typically from a dentist (55.3%) or pediatrician (39.7%).
Prescriber types influencing duration of use
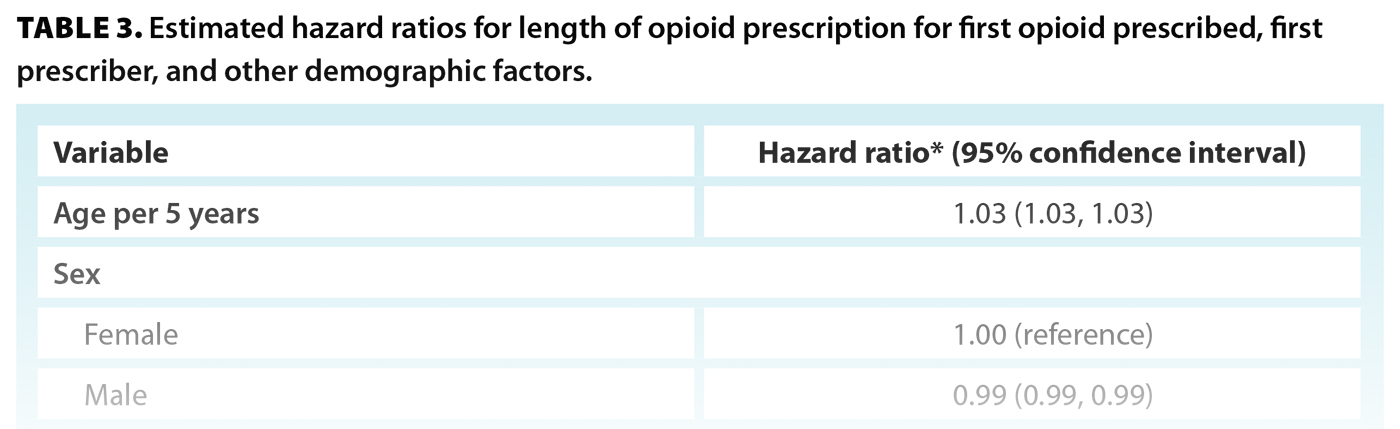
Aa a first prescriber specialty type, psychiatry was associated with a 32% longer episode duration compared with family practice [Table 3]. Other specialty types with longer episode durations included pharmacy, nurse practitioner, and diagnostic medicine. Dentistry (oral surgery), dentistry, surgery, and pediatrics were associated with shorter episode durations.
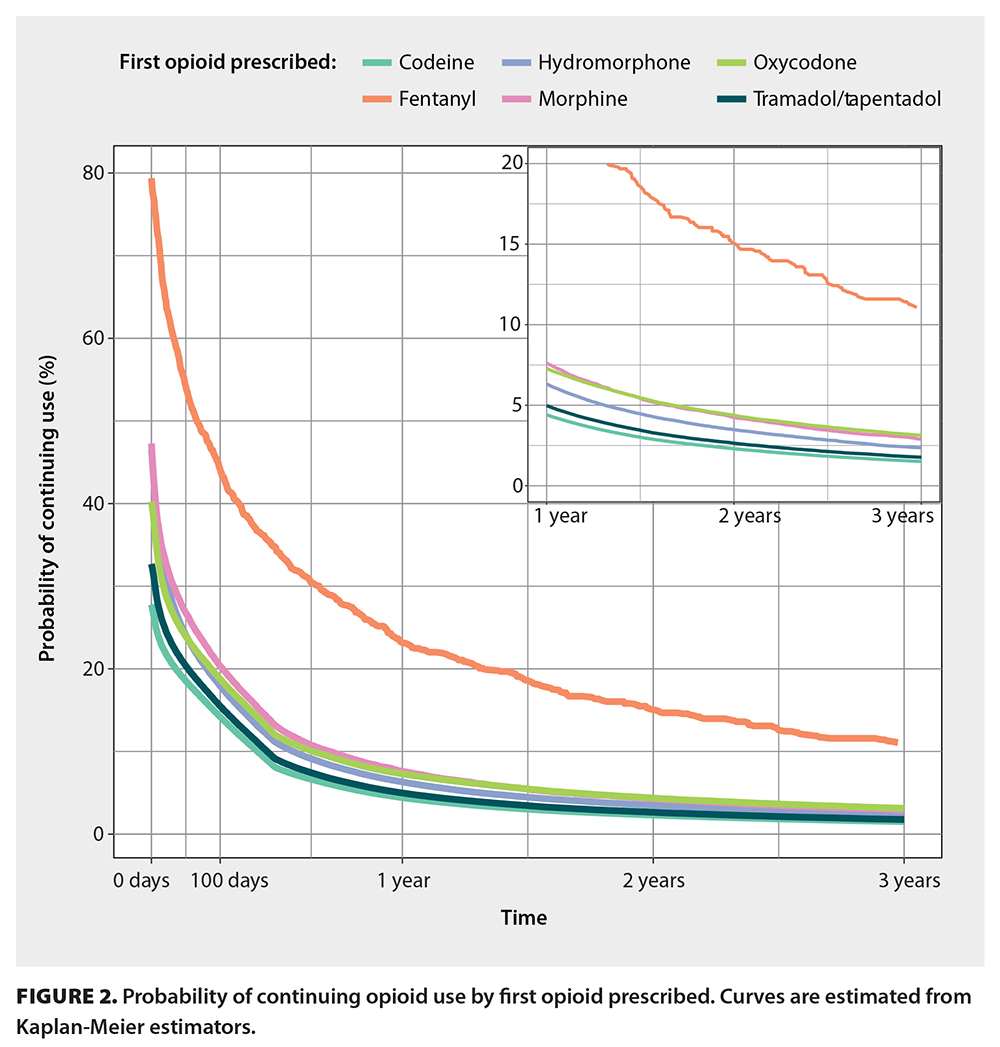 First opioid prescription type influencing duration of use
First opioid prescription type influencing duration of use
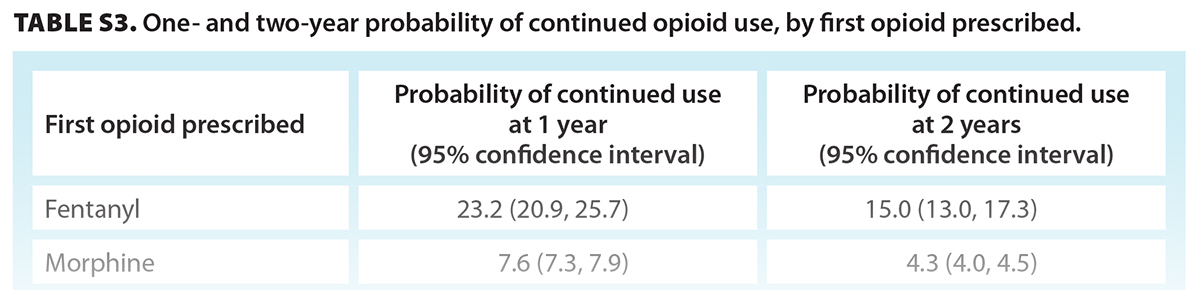
Episodes with a first opioid prescription of butorphanol or fentanyl were 60% longer than episodes that began with a prescription of codeine [Table 3]. Episodes with a first prescription of tramadol/tapentadol or pentazocine were similar in duration (2% to 4% longer) to episodes that began with a prescription of codeine. Users who were first prescribed fentanyl had a 23.2% probability of continued use after 1 year and a 15.0% probability after 2 years [Figure 2, Table S3]. Individuals who were first prescribed codeine, tramadol/tapendatol, hydromorphone, oxycodone, or morphine had a 4.4% to 7.6% probability of continued use at 1 year and a 2.3% to 4.4% probability at 2 years [Table S3].
Discussion
Over the 4-year study period, 7.2% of opioid-naive individuals became new long-term users. Compared with non-long-term users, new long-term users were prescribed a lower initial MME (22.5 vs 30.0) but with a longer supply (10 vs 5 days), which meant they received a higher total initial opioid dose (225 vs 150 MME). New long-term users most often received their prescription from a family physician (79.5%) compared with non-long-term users (48.7%). Almost one-quarter of long-term episodes involved three or more different opioid types, and nearly one-fifth involved prescriptions from three or more prescriber types. At the end of the study period, 59.8% of new long-term users had stopped opioid use, 37.9% had ongoing opioid use (opioid other than methadone or buprenorphine), and 2.3% had transitioned to methadone or buprenorphine.
Prescriptions
Our study population was similar to that of Jani and colleagues[20] in terms of sample size and age and sex distribution. Although we found a higher initial prescription MME in non-long-term users compared with new long-term users, this finding is difficult to explain without knowing the indication for use. The median MME of the initial prescription for new long-term users (22.5 MME) was lower than the initial prescribing opioid dose in other North American regions (38.0 MME in Boston), similar to that in other Canadian provinces (27.0 MME in Quebec; 23.0 MME in Alberta), and higher than that in other areas of the world (12.0 MME in the United Kingdom; 8.0 MME in Taiwan).[20] These geographical variations have been attributed, in part, to the type of opioid prescribed: codeine is more commonly prescribed in Canada and the United Kingdom, whereas oxycodone and tramadol are more commonly initially prescribed in Boston and Taiwan, respectively.[20] In Portugal, which has low opioid use and opioid-related deaths compared with other countries in the Organisation for Economic Co-operation and Development, tramadol, buprenorphine, and tapentadol are the three most prescribed opioids for any indication.[32] In our study, codeine and tramadol were prescribed more than any other opioid across new long-term users and non-long-term users. This may reflect the relative ease with which physicians can prescribe these opioids. Stronger opioids required a duplicate prescription during the study period. Additionally, opioid-naive individuals who became new long-term users received an initial prescription with a longer supply compared with non-long-term users (10 vs 5 days). This finding is consistent with that of Shah and colleagues, who found that increasing the days supplied was associated with a higher likelihood of continued opioid use.[12] In contrast to Shah and colleagues, who found that patients who initially received tramadol were less likely to discontinue opioid use, our results showed that individuals who were prescribed fentanyl, morphine, or oxycodone had the higher probability of continued use at 2 years. Although we found that hydromorphone and oxycodone were associated with longer episode lengths, it is difficult to hypothesize why this may be in the absence of knowing the indications for prescribing.
Prescribers
Our study is unique in that we characterized opioid prescribers by opioid user type. Nearly 80% of prescribers for new long-term users were from family practice. In contrast, only 50% of prescribers in the non-long-term user group were from family practice. Surgery and dentistry accounted for 18.1% and 15.6% of the remaining initial prescriptions, respectively. The almost 20% of new long-term users with three or more prescriber types may be a reflection of patients who lacked a primary care provider; had complex medical problems; went to walk-in clinics, emergency departments, or pain clinics; and/or had opioid-seeking behavior. Moreover, prescribers are not required to review a patient’s past prescriptions.[33] Our finding of family physicians prescribing opioids to long-term users is similar to that in Germany and France but differs from regions in Northern Europe where opioids for chronic pain are prescribed mainly by specialists.[32] Additionally, other regions, such as Portugal, have Pharmacy and Therapeutics Committees that are responsible by law to monitor prescription patterns, provide recommendations, and advance research.[32] To date, nothing similar exists in Canada.
Although the first prescriber type for new long-term users was family physician (80%), it is important to contextualize this finding within the limitations of the study data and health care system. Data were limited to outpatient prescriptions; therefore, an initial prescription within the study data may have been a renewal of a prescription prescribed in a hospital setting (e.g., emergency department, hospital prescriptions). Within BC’s health care system, long-term follow-up care often circles back to the family physician (or, less often, a psychiatrist or another clinician). If a treatment plan is unclear, this may limit a family physician’s ability to appropriately evaluate a patient’s request to continue a prescription.[34]
In our study, longer durations of opioid use were associated with prescribers from certain specialties: psychiatry, pharmacy, nurse practitioner, or diagnostic medicine, whereas oral surgery, dentistry, pediatrics, and surgery were associated with shorter episode durations. This may reflect pain etiology.
Long-term opioid therapy
Because we did not have access to the indication for each prescription, we were unable to determine which prescriptions were appropriate for long-term opioid therapy and which may have been associated with an opioid use disorder. Long-term opioid therapy may be appropriate pain management for some individuals; however, the benefits of long-term opioid use for noncancer chronic pain have been questioned due to concerns about side effect profiles, opioid use disorder, diversion, overdose, and death. In 2020 and 2021, 24% and 21%, respectively, of all US opioid deaths involved a prescription opioid.[35] Due to the pharmacological properties of opioids, repeat dosing results in tolerance and dependence, and a small percentage of individuals may develop addiction, even with short-term use.[36] With higher doses of opioids prescribed to individuals in the community, the probability of diversion increases, with the most common form being patients sharing their prescribed opioids with their family or friends for self-medication purposes.[36]
Methadone/buprenorphine
Most new long-term users in our study did not receive methadone or buprenorphine. This may be partially explained by the fact that we were not able to account for other agents that are currently used for opioid agonist therapy, such as slow-release morphine (Kadian), which has been commonly prescribed since 2015–2016.Our findings indicate that a number of previously opioid-naive individuals prescribed long-term opioid therapy (non-opioid agonist) may have benefited from a weaning schedule or conversion to opioid agonist therapy. Without knowing the indication for opioid prescription, it is difficult to make firm recommendations. It is clear that long-term opioid therapy for noncancer pain is associated with risks; current advice is to help patients wean off the opioid or use the lowest dose that helps them function in daily life.[37] However, weaning management alone is not appropriate without providing suitable multidisciplinary support and other alternatives for pain management. Deprescribing or weaning management alone is associated with harm such as withdrawal, depression, suicide ideation, and seeking illicit drugs for opioid replacement, with its antecedent risks of overdose, illicit drug-related infections, and death.[38] Other supportive measures may be indicated, including referral to ongoing addiction therapy (e.g., intensive outpatient treatment, residential treatment); provider-led counseling; long-term substance use monitoring (e.g., regular assessment, follow-up, and urine drug tests); comprehensive preventive and primary care; and psychological treatment interventions, social supports, and specialist care.[39] A better awareness of nonprescription and prescription factors for overdoses, recently described by Wang and colleagues,[18] and the first opioid prescription variables that contribute to longer durations of opioid use may facilitate shared decision making regarding prescribing opioids for chronic pain and may inform harm-reduction strategies.
Study limitations
Our study focused on community prescribing practices alone and did not include hospital prescriptions. We were unable to account for the indication of prescription and/or pain etiology across user types, so we cannot interpret the appropriateness of opioid prescriptions—for example, opioid prescriptions as part of cancer treatment. For the same reason, we cannot make inferences about whether patients were being prescribed opioids for opioid use disorder. The lack of indications for these prescriptions leaves gaps in our understanding and ability to make recommendations. Furthermore, our study did not include any outcome data; thus, we are unable to determine why a patient may have stopped taking opioids or why an episode ended. Additionally, we cannot confirm that opioids were used as prescribed or the fate of any unused opioids. Because our study did not include hospital prescriptions, we do not know how many opioid-naive patients had hospitalizations or emergency room visits and received opioids prior to discharge and their first community-dispensed opioid prescription. It is possible our definition of opioid-naive may have included individuals who were not truly opioid-naive—for example, patients who had recently moved to BC or who had been discharged from hospital care. First prescriptions may have been a renewal of a hospital prescription. Thus, our study may have overestimated the number of first prescriptions provided by family physicians. In terms of prescriptions, it is likely the days supplied calculated from “as needed” prescriptions were unreliable. We were also unable to account for slow-release drug formulations. We did not examine whether prescriptions from multiple prescribers overlapped, whether combination agents (e.g., oxycodone/naloxone) or additional substances (e.g., benzodiazepines) were used, or how these factors may have affected the risk of long-term use. Despite these limitations, our long-term study over 4 years gives a better understanding of how opioid prescribing evolves with time, which enables informed decision making and policy to promote safe prescribing that is aligned with patient needs.
Conclusions
In summary, 7.2% of previously opioid-naive individuals became new long-term users over the 4-year study period. Longer duration of opioid use was associated with older age, a first prescription of fentanyl or butorphanol, and a first prescriber specialty type of psychiatry. In this cohort, only 5.7% of new long-term opioid users received a prescription for methadone or buprenorphine. It would be interesting to see how this proportion may have changed in the following years, from 2018 to 2023. Without data on indications and outcomes, it is challenging to determine the appropriateness of the prescriptions analyzed; however, our findings suggest that further guidance on prescribing practices is necessary. Changing opioid prescription practices is key in reducing opioid overdose.[11] Primary care providers, as the main prescribers of opioids, are in a key position to prevent long-term opioid use. North American opioid guidelines have had a significant effect on reducing opioid prescriptions, but our work suggests that more guidance may be required to target long-term opioid therapy in BC. Emerging evidence of an association between precipitous opioid tapering with symptoms of withdrawal, mental health issues, suicide, and overdose compounds this issue.[38] Hence, evidence-informed measures that focus on effective—but safe—reduction and cessation of prescribed opioids in primary care are needed. Future work that builds on our findings by incorporating indications would be a valuable contribution to our understanding of patterns in long-term opioid therapy prescribing.
Availability of data and material
Access to data provided by the data stewards is subject to approval but can be requested for research projects through the data stewards or their designated service providers. The following data sets were used in this study: PharmaNet and Medical Services Plan Practitioner File. You can find further information on these data sets by visiting the PopData project web page at https://my.popdata.bc.ca/project_listings/18-216/collection_approval_dates. All inferences, opinions, and conclusions in this article are those of the authors and do not reflect the opinions or policies of the data stewards.
Code availability
Please contact the corresponding author for further information.
Acknowledgments
The authors would like to thank Dr Marija Bucevska, Ms Young Ji Tuen, and Ms Sophia Shayan for their assistance coordinating this project. We would also like to thank the University of British Columbia Division of Plastic Surgery, the UBC Faculty of Medicine, and the BC Children’s Hospital Research Institute for funding this work.
Funding
This study was funded by a BC Children’s Hospital Research Institute Evidence to Innovation Theme Seed Grant Award (grant number GR019197 BCCHR) and by a University of British Columbia Faculty of Medicine Summer Student Research Program grant awarded to R.X.
Competing interests
None declared.
This article has been peer reviewed.
 |
| This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. |
References
1. Paul AK, Smith CM, Rahmatullah M, et al. Opioid analgesia and opioid-induced adverse effects: A review. Pharmaceuticals 2021;14:1091. https://doi.org/10.3390/ph14111091.
2. Manhapra A, Sullivan MD, Ballantyne JC, et al. Complex persistent opioid dependence with long-term opioids: A gray area that needs definition, better understanding, treatment guidance, and policy changes. J Gen Intern Med 2020;35(Suppl 3):964-971. https://doi.org/10.1007/s11606-020-06251-w.
3. Häuser W, Schubert T, Vogelmann T, et al. All-cause mortality in patients with long-term opioid therapy compared with non-opioid analgesics for chronic non-cancer pain: A database study. BMC Med 2020;18:162. https://doi.org/10.1186/s12916-020-01644-4.
4. Scherrer JF, Salas J, Miller-Matero LR, et al. Long-term prescription opioid users’ risk for new-onset depression increases with frequency of use. Pain 2022;163:1581-1589. https://doi.org/10.1097/j.pain.0000000000002547.
5. Mojtabai R. National trends in long-term use of prescription opioids. Pharmacoepidemiol Drug Saf 2018;27:526-534. https://doi.org/10.1002/pds.4278.
6. Canadian Institute for Health Information. Opioid prescribing in Canada: How are practices changing? 2019. Accessed 26 January 2024. www.cihi.ca/sites/default/files/document/opioid-prescribing-canada-trends-en-web.pdf.
7. Centers for Disease Control and Prevention. 2018 annual surveillance report of drug-related risks and outcomes—United States. Surveillance special report. 2018. Accessed 26 January 2024. www.cdc.gov/overdose-prevention/media/pdfs/pubs/2018-cdc-drug-surveillance-report.pdf.
8. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: A systematic review and data synthesis. Pain 2015;156:569-576. https://doi.org/10.1097/01.j.pain.0000460357.01998.f1.
9. BC Coroners Service. Statistical reports on deaths in British Columbia. Unregulated drug deaths in BC (to April 30, 2023). Accessed 6 June 2023. www2.gov.bc.ca/gov/content/life-events/death/coroners-service/statistical-reports.
10. Public Safety and Solicitor General. Toxic-drug supply claims nearly 2,300 lives in 2022: BC Coroners Service. Accessed 6 June 2023. https://news.gov.bc.ca/releases/2023PSSG0008-000109.
11. Bricker DA, Crawford TN, Castle A, et al. PRESTO: Promoting engagement for the safe tapering of opioids. Pain 2023;164:2553-2563. https://doi.org/10.1097/j.pain.0000000000002961.
12. Shah A, Hayes CJ, Martin BC. Factors influencing long-term opioid use among opioid naive patients: An examination of initial prescription characteristics and pain etiologies. J Pain 2017;18:1374-1383. https://doi.org/10.1016/j.jpain.2017.06.010.
13. Lyden J, Binswanger IA. The United States opioid epidemic. Semin Perinatol 2019;43:123-131. https://doi.org/10.1053/j.semperi.2019.01.001.
14. Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants – United States, 2015–2016. MMWR 2018;67:349-358. https://doi.org/10.15585/mmwr.mm6712a1.
15. Deyo RA, Hallvik SE, Hildebran C, et al. Association between initial opioid prescribing patterns and subsequent long-term use among opioid-naïve patients: A statewide retrospective cohort study. J Gen Intern Med 2017;32:21-27. https://doi.org/10.1007/s11606-016-3810-3.
16. Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use – United States, 2006–2015. MMWR 2017;66:265-269. https://doi.org/10.15585/mmwr.mm6610a1.
17. Miller M, Barber CW, Leatherman S, et al. Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med 2015;175:608-615. https://doi.org/10.1001/jamainternmed.2014.8071.
18. Wang L, Hong PJ, Jiang W, et al. Predictors of fatal and nonfatal overdose after prescription of opioids for chronic pain: A systematic review and meta-analysis of observational studies. CMAJ 2023;195:E1399-E1411. https://doi.org/10.1503/cmaj.230459.
19. Mclaughlin S, Overell J, Rossaak J. Opioid prescribing patterns following common general surgery procedures in the Bay of Plenty, New Zealand. ANZ J Surg 2023;93:597-601. https://doi.org/10.1111/ans.18319.
20. Jani M, Girard N, Bates DW, et al. Opioid prescribing among new users for non-cancer pain in the USA, Canada, UK, and Taiwan: A population-based cohort study. PLoS Med 2021;18:e1003829. https://doi.org/10.1371/journal.pmed.1003829.
21. Hill MV, McMahon ML, Stucke RS, Barth RJ Jr. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg 2017;265:709-714. https://doi.org/10.1097/SLA.0000000000001993.
22. Hill MV, Stucke RS, Billmeier SE, et al. Guideline for discharge opioid prescriptions after inpatient general surgical procedures. J Am Coll Surg 2018;226:996-1003. https://doi.org/10.1016/j.jamcollsurg.2017.10.012.
23. Lewis ET, Cucciare MA, Trafton JA. What do patients do with unused opioid medications? Clin J Pain 2014;30:654-662. https://doi.org/10.1097/01.ajp.0000435447.96642.f4.
24. Nann P, Nabavi N, Ziafat K, et al. Trends in opioid dispensing after common abdominal and orthopedic surgery procedures in British Columbia: A retrospective cohort analysis. Can J Anaesth 2022;69:986-996. https://doi.org/10.1007/s12630-022-02272-7.
25. Yefet LS, Bone JN, Courtemanche R, et al. Opioid prescribing patterns in British Columbia from 2013 to 2017: A population-based study. BCMJ 2021;63:336-342.
26. Population Data BC. Publishing research materials and data steward review requirements. Accessed 8 May 2024. www.popdata.bc.ca/data_access/publishing_research_materials.
27. Centers for Disease Control and Prevention. Data resources. 2019. Accessed 20 May 2020. www.cdc.gov/overdose-prevention/data-research/facts-stats/index.html. This file has now been discontinued: https://archive.cdc.gov/#/details?q=US%20Centers%20for%20Disease%20Control%E2%80%99s%202017%20oral%20MME%20guide&start=0&rows=10&url=https://www.cdc.gov/opioids/data-resources/index.html.
28. O’Connor J, Christie R, Harris E, et al. Tramadol and tapentadol: Clinical and pharmacologic review. 2019. World Federation of Societies of Anaesthesiologists. Published 23 July 2019. Accessed 17 July 2021. https://resources.wfsahq.org/atotw/tramadol-and-tapentadol-clinical-and-pharmacologic-review.
29. Population Data BC. Medical Services Plan (MSP) data set (permission required). Vancouver: University of British Columbia. Accessed 2020. www.popdata.bc.ca/data.
30. Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain 2008;24:521-527. https://doi.org/10.1097/AJP.0b013e318169d03b.
31. R Foundation for Statistical Computing. The R project for statistical computing. Version 4.0. Accessed 14 February 2024. www.r-project.org.
32. Caldeira D, Broeiro P, Cimadeira F, et al. Opioids prescribing trend between 2013 and 2017 in the Lisbon and Tagus Valley region, Portugal. Int J Clin Pharm 2021;43:323-327. https://doi.org/10.1007/s11096-020-01199-7.
33. Population Data BC. PharmaNet data set. Accessed 31 January 2024. www.popdata.bc.ca/data/health/pharmanet.
34. Desveaux L, Saragosa M, Kithulegoda N, Ivers NM. Understanding the behavioural determinants of opioid prescribing among family physicians: A qualitative study. BMC Fam Pract 2019;20:59. https://doi.org/10.1186/s12875-019-0947-2.
35. National Institute on Drug Abuse. Drug overdose deaths: Facts and figures. National Institutes of Health. Accessed 29 January 2024. https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates.
36. Volkow ND, McLellan AT. Opioid abuse in chronic pain—Misconceptions and mitigation strategies. N Engl J Med 2016;374:1253-1263. https://doi.org/10.1056/NEJMra1507771.
37. Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ 2017;189:E659-E666. https://doi.org/10.1503/cmaj.170363.
38. Fenton JJ, Magnan E, Tseregounis IE, et al. Long-term risk of overdose or mental health crisis after opioid dose tapering. JAMA Netw Open 2022;5:e2216726. https://doi.org/10.1001/jamanetworkopen.2022.16726.
39. British Columbia Centre on Substance Use. A guideline for the clinical management of opioid use disorder: 2023 update. Victoria: British Columbia Centre on Substance Use, BC Ministry of Health, and BC Ministry of Mental Health and Addictions, 2023. Accessed 2 December 2024. www.bccsu.ca/wp-content/uploads/2023/12/BC-OUD-Treatment-Guideline_2023-Update.pdf.
Dr Xu is a plastic surgery resident in the Division of Plastic, Reconstructive and Aesthetic Surgery in the Department of Surgery at the University of Toronto (ORCID ID: 0000-0003-3617-1031). Dr Bone is a biostatistical lead in the Clinical Research Support Unit at the BC Children’s Hospital Research Institute (ORCID ID: 0000-0001-7704-1677). Ms Courtemanche is the clinical research manager for the Division of Plastic Surgery at the University of British Columbia (ORCID ID: 0000-0002-8805-3670). Dr Yefet is a neurosurgery resident in the Division of Neurosurgery in the Department of Surgery at the University of Toronto (ORCID ID: 0000-0003-1504-1772). Dr Simmonds is a consultant pediatric anesthetist at Women’s and Children’s Hospital in Adelaide, Australia. Dr Cattoni is a family physician in the Sheway medical clinic in Vancouver; the medical director of FIR Square, BC Women’s Hospital and Health Centre; a consultant physician in the Perinatal Addictions Service, BC Women’s Hospital and Health Centre; and a clinical assistant professor in the Faculty of Medicine at UBC. Dr Lauder is a pediatric anesthesiologist and a clinical professor in the Faculty of Medicine at UBC and the Department of Pediatric Anesthesia at BC Children’s Hospital (ORCID ID: 0000-0001-6774-6120). Dr Courtemanche is a clinical professor in the Department of Surgery at UBC and the Division of Pediatric Plastic Surgery at BC Children’s Hospital (ORCID ID: 0000-0003-3720-9302).
Corresponding author: Dr Douglas J. Courtemanche, douglas.courtemanche@ubc.ca.