Clinical Case Reports
Mycobacterium heckeshornense tenosynovitis: Case report and literature review
ABSTRACT: Mycobacterium heckeshornense is not a commonly isolated organism in British Columbia. We present the first known case of M. heckeshornense tenosynovitis in BC, in the hand of an immunocompetent patient. M. heckeshornense was identified using hsp65 gene sequencing, and treatment consisted of surgery and multidrug antimycobacterial therapy. Nontuberculous mycobacteria should be considered as a potential cause of culture-negative tenosynovitis. Because the number of cases implicating M. heckeshornense continues to rise, it is important to correctly identify the organism and differentiate it from the phylogenetically related M. xenopi. More research and treatment experience are needed before a management guideline for M. heckeshornense can be developed.
Mycobacterium heckeshornense should be considered in all patients who present with atypical joint infections, because most infections occur in immunocompetent patients and patients rarely report specific exposures.
Case data
A 91-year-old man was assessed by the infectious diseases service at Surrey Memorial Hospital after 3 months of swelling and erythema in the third digit of his left hand. He reported spontaneous development of a small papule that developed into an ulcerating lesion in the volar aspect of the finger around the distal interphalangeal and metacarpophalangeal joints. There was associated dactylitis. He was systemically well with no fevers or chills. He reported no trauma, animal bites or scratches, freshwater or saltwater exposure, or travel outside of Canada. His medical history included gout, diabetes, hypothyroidism, dyslipidemia, chronic kidney disease, congestive heart failure, benign prostatic hypertrophy, and right temporal stroke. His regular medications included insulin, atorvastatin, ferrous fumarate, levothyroxine, carvedilol, aspirin, and hydralazine. He had no known drug allergies.
The patient received courses of oral cloxacillin, intravenous cefazolin, and ceftriaxone for cellulitis and suspected septic arthritis. Joint aspiration revealed minimal fluid. Crystal analysis and cell count and differential could not be performed. Bacterial culture was negative. He was treated with steroids for a possible gout flare and seemed to have partial response.
The joint was re-aspirated 1 month later, which showed 278 000 × 106/L total nucleated cells with 92% neutrophils. Crystal analysis and bacterial cultures were negative. Given the subacute clinical presentation, fungal and mycobacterial cultures were obtained. They revealed 1+ acid-fast bacilli on auramine-rhodamine staining. Molecular testing for M. tuberculosis and M. avium complex were negative. The sample was inoculated on liquid and solid media for mycobacterial culture. Throughout the patient’s presentation, his white cell count and differential remained normal, and his C-reactive protein was only mildly elevated, with a peak of 22.7 mg/L.
 The patient developed worsening seropurulent discharge from the affected finger and underwent incision and drainage. Operative cultures were positive for a few colonies of methicillin-resistant Staphylococcus aureus. The postoperative wound is shown in Figure 1. Histopathology showed granulation tissue and fibrinopurulent exudate, with negative periodic acid-Schiff and acid-fast bacilli stains. A deep wound swab was negative for acid-fast bacilli and had no growth in mycobacterial culture at 8 weeks of incubation. The patient received vancomycin, then subsequent daptomycin therapy for possible methicillin-resistant S. aureus tenosynovitis.
The patient developed worsening seropurulent discharge from the affected finger and underwent incision and drainage. Operative cultures were positive for a few colonies of methicillin-resistant Staphylococcus aureus. The postoperative wound is shown in Figure 1. Histopathology showed granulation tissue and fibrinopurulent exudate, with negative periodic acid-Schiff and acid-fast bacilli stains. A deep wound swab was negative for acid-fast bacilli and had no growth in mycobacterial culture at 8 weeks of incubation. The patient received vancomycin, then subsequent daptomycin therapy for possible methicillin-resistant S. aureus tenosynovitis.
Despite 5 weeks of daptomycin therapy, the patient developed a new ulcerating lesion in the volar aspect of his finger, with surrounding hypergranulating tissue and serous drainage. Daptomycin was discontinued because there was no evidence of improvement, and repeat tissue cultures were obtained. Bacterial culture, acid-fast bacilli smear, and mycobacterial culture were negative.
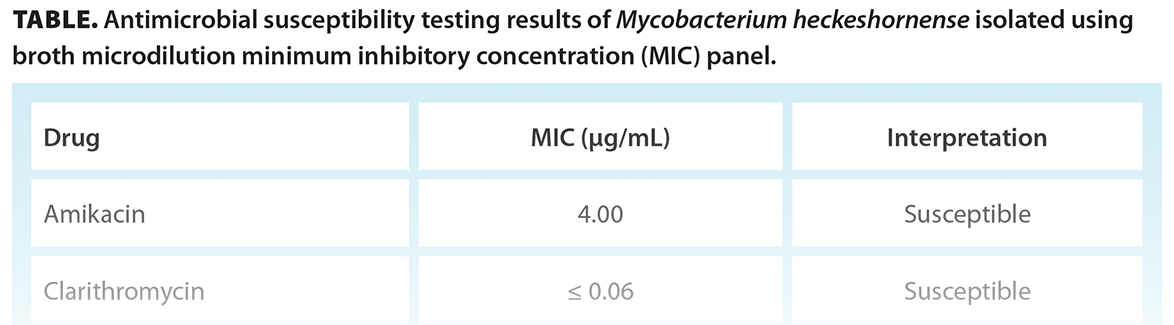
The mycobacterial liquid culture (BACTEC MGIT 960 system) of the original acid-fast bacilli smear-positive aspirate flagged as positive at 5 weeks of incubation. Further incubation at 6 weeks demonstrated small yellow colonies on Löwenstein-Jensen solid medium, with short to medium acid-fast bacilli observed on smear. The organism was identified as M. heckeshornense using hsp65 gene sequencing. Susceptibility testing results were obtained from the National Microbiology Laboratory in Winnipeg [Table].
The patient underwent further debridement and left third-finger tenolysis for chronic flexor tenosynovitis. Operative samples were negative for mycobacteria at 6 weeks of incubation. Pathological review showed granulation tissue with abscess formation. Periodic acid-Schiff and acid-fast bacilli stains were negative, and the sample was negative for malignancy. The patient was started on treatment with clarithromycin, moxifloxacin, and isoniazid based on susceptibility results.
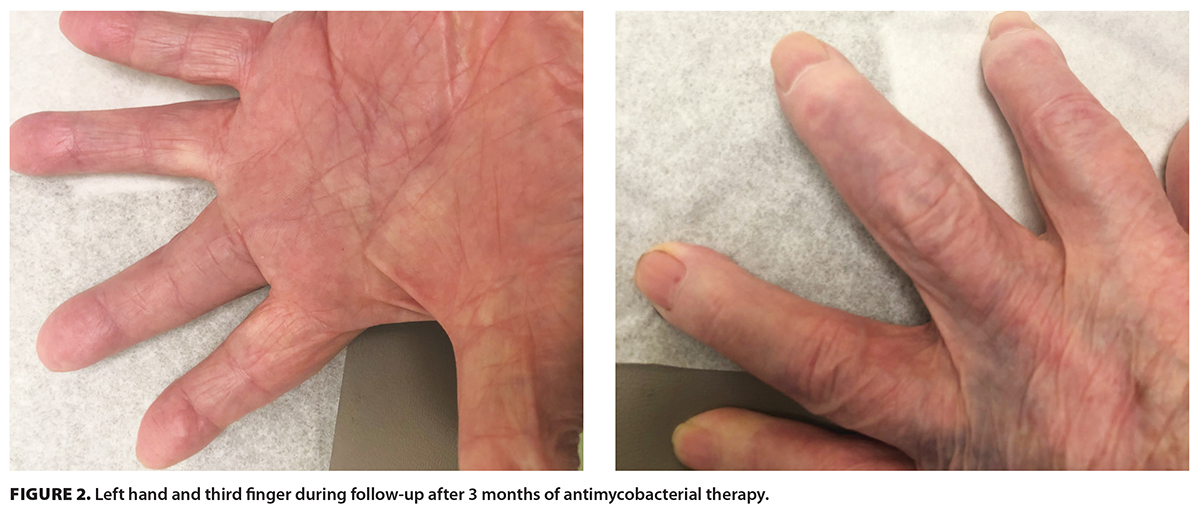
The patient was reassessed in the outpatient infectious diseases clinic approximately 3 months after starting antimycobacterial therapy. The swelling had decreased, and there had been no recurrent ulcers since the last tenolysis [Figure 2]. The plan was to continue combination therapy for 9 to 12 months if there was ongoing improvement. If the patient had poor clinical response, further consideration would be given to digit amputation by plastic surgery. However, the patient presented to hospital the following month with an unrelated issue of subdural hematoma and died.
Discussion
Hands and wrists are common sites of nontuberculous mycobacterial infections due to a higher likelihood of penetrating injuries.[1] The most common species implicated are M. marinum, M. chelonae, M. kansasii, and M. intracellulare. Fifty-three percent of nontuberculous mycobacterial infections of the hand are initially misdiagnosed, leading to delayed treatment and potentially advanced infection by the time they are recognized.[1] Notably, serum inflammatory markers are usually normal, as described in our case. It is not known how our patient was exposed to M. heckeshornense.
M. heckeshornense is a rare nontuberculous mycobacterium that is phylogenetically related to M. xenopi.[2] It was first identified in 2000 at the Heckeshorn Lung Clinic in Berlin in respiratory mycobacterial cultures of an immunocompetent woman with chronic cavitary lung lesions.[2] Pulmonary and extrapulmonary infections by this organism have been described around the world, with varied clinical outcomes.[2-19] Extrapulmonary manifestations include peritonitis,[3] lumbar spondylodiscitis/osteomyelitis,[4-6] axillary lymphadenitis,[7] bacteremia,[8] and synovitis.[9] In the one published case of M. heckeshornense tenosynovitis, surgical treatment alone with flexor tenosynovectomy was effective.[10]
Most M. heckeshornense infections occur in immunocompetent patients. For this reason, M. heckeshornense and other nontuberculous mycobacterial infections should be considered in all patients who present with atypical joint infections. Patients rarely report specific exposures, which supports the theory that M. heckeshornense is widely distributed.
As with other mycobacteria, microbiological confirmation of M. heckeshornense infection may be challenging, because the organisms may not be found in all parts of the affected tissues (as evidenced by recovery of this organism in only one of the multiple specimens collected from our patient). Tissue or fluid, rather than swabs, are the appropriate sample types for mycobacterial smear and culture. M. heckeshornense is a slow-growing scotochromogen that is able to grow in temperatures ranging from 37 °C to 45 °C, but not at 30 °C.[2] Sequencing of both 16S rRNA and hsp65 regions can be used to identify M. heckeshornense.[10] Granulomata, giant cells, rice bodies, and central necrosis are supportive histopathological clues. In our case, M. heckeshornense was ultimately recovered in the aspiration sample obtained after the patient had received a course of steroid therapy. Therefore, it is possible that initial treatment with steroids suppressed the patient’s immune system sufficiently to create an environment suitable for this nontuberculous mycobacterium to flourish.
There are no established guidelines for the treatment of M. heckeshornense. The literature supports management with surgical source control and the use of combination antimicrobials.[3] It is important to consider in vitro susceptibilities, because M. heckeshornense has been found to have reduced susceptibilities to isoniazid and may acquire resistance to rifampicin with long-term treatment.[2] However, in vitro susceptibility results for nontuberculous mycobacteria do not have optimal correlation with clinical response. In many cases, despite the use of combination antimicrobials, definitive adjunctive treatment with surgical source control is required for the resolution of symptoms. There is no consensus on the choice and duration of antimicrobial treatment for nontuberculous mycobacterial hand infections, which often ranges from 6 to 12 months but can sometimes be more prolonged.[1]
M. heckeshornense is not a commonly isolated organism in BC. Over the last decade, it has been confirmed in less than five cases per year, with most identified in respiratory specimens. The clinical significance of M. heckeshornense in these patients is unknown. Among clinically significant cases in BC that we know about, M. heckeshornense was described as a source of peritoneal dialysis–associated peritonitis in 2011, where source control with peritoneal catheter removal and fluid drainage alone was adequate for disease resolution,[3] and an unpublished case of a M. heckeshornense bacteremia in an immunocompromised patient was diagnosed in 2020.
Summary
We present a case of M. heckeshornense tenosynovitis in an immunocompetent patient. As the number of cases implicating M. heckeshornense continues to rise, it is important to correctly identify this mycobacterium and differentiate it from the phylogenetically related M. xenopi. More research and treatment experience are needed before a management guideline for M. heckeshornense can be developed.
Competing interests
None declared.
This article has been peer reviewed.
 |
| This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. |
References
1. Balagué N, Uçkay I, Vostrel P, et al. Non-tuberculous mycobacterial infections of the hand. Chir Main 2015;34:18-23. https://doi.org/10.1016/j.main.2014.12.004.
2. Roth A, Reischl U, Schönfeld N, et al. Mycobacterium heckeshornense sp. nov., a new pathogenic slowly growing Mycobacterium sp. causing cavitary lung disease in an immunocompetent patient. J Clin Microbiol 2000;38:4102-4107. https://doi.org/10.1128/JCM.38.11.4102-4107.2000.
3. Chan WW, Murray MC, Tang P, Romney MG. Mycobacterium heckeshornense peritonitis in a peritoneal dialysis patient: A case report and review of the literature. Clin Microbiol Infect 2011;17:1262-1264. https://doi.org/10.1111/j.1469-0691.2010.03449.x.
4. Elyousfi AA, Leiter JRS, Goytan MJ, Robinson DB. Mycobacterium heckeshornense lumbar spondylodiskitis in a patient with rheumatoid arthritis receiving etanercept treatment. J Rheumatol 2009;36:2130-2131. https://doi.org/10.3899/jrheum.090056.
5. Carpenter RJ, Graf PCF. Pott’s disease? AIDS-associated Mycobacterium heckeshornense spinal osteomyelitis and diskitis. J Clin Microbiol 2015;53:716-718. https://doi.org/10.1128/JCM.02686-14.
6. Douiri N, Lefebvre N, Hansmann Y, et al. Relapsing Pott disease caused by Mycobacterium heckeshornense in a well-controlled HIV-infected patient. Med Mal Infect 2018;48:157-158. https://doi.org/10.1016/j.medmal.2017.12.003.
7. McBride SJ, Taylor SL, Pandey SK, Holland DJ. First case of Mycobacterium heckeshornense lymphadenitis. J Clin Microbiol 2009;47:268-270. https://doi.org/10.1128/JCM.00890-08.
8. Ahmed RA, Miedzinski LJ, Shandro C. Mycobacterium heckeshornense infection in HIV-infected patient. Emerg Infect Dis 2010;16:1801-1803. https://doi.org/10.3201/eid1611.091226.
9. Nicolás-de Blas R, Garijo-Bufort M, Nebreda-Mayoral T, Guerra-Laso JM. Synovitis due to Mycobacterium heckeshornense in a patient with rheumatoid arthritis and treatment with infliximab. Enferm Infecc Microbiol Clin (English ed.) 2020;38:448-449. https://doi.org/10.1016/j.eimc.2020.01.011.
10. Godreuil S, Marchandin H, Terru D, et al. Myco-bacterium heckeshornense tenosynovitis. Scand J Infect Dis 2006;38:1098-1101. https://doi.org/10.1080/00365540600606606.
11. van Hest R, van der Zanden A, Boeree M, et al. Mycobacterium heckeshornense infection in an immunocompetent patient and identification by 16S rRNA sequence analysis of culture material and a histopathology tissue specimen. J Clin Microbiol 2004;42:4386-4389. https://doi.org/10.1128/JCM.42.9.4386-4389.2004.
12. Jauréguy F, Ioos V, Marzouk P, et al. Mycobacterium heckeshornense: An emerging pathogen responsible for a recurrent lung infection. J Infect 2007;54:e33-e35. https://doi.org/10.1016/j.jinf.2006.03.026.
13. Morimoto K, Kazumi Y, Maeda S, et al. Mycobacterium heckeshornense lung infection that was diagnosed as Mycobacterium xenopi disease by DNA-DNA hybridization (DDH). Intern Med 2011;50:1251-1253. https://doi.org/10.2169/internalmedicine.50.4628.
14. Coitinho C, Greif G, van Ingen J, et al. First case of Mycobacterium heckeshornense cavitary lung disease in the Latin America and Caribbean region. New Microbes New Infect 2015;9:63-65. https://doi.org/10.1016/j.nmni.2015.12.003.
15. Kurosaki F, Yoshimoto T, Nakayama M, et al. Pulmonary Mycobacterium heckeshornense infection in a healthy woman. J Infect Chemother 2018;24:483-486. http://doi.org/10.1016/j.jiac.2018.01.006.
16. Iitoh E, Tominaga M, Okamoto M, et al. A case of pulmonary Mycobacterium heckeshornense infection in a healthy Japanese man. Respir Med Case Rep 2020;30:101093. https://doi.org/10.1016/j.rmcr.2020.101093.
17. Yokoyama A, Kage H, Ohama Y, et al. Mycobacterium heckeshornense lung infection diagnosed by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). J Infect Chemother 2018;24:763-765. https://doi.org/10.1016/j.jiac.2018.01.017.
18. Howell D, Galen BT. Recurrent rheumatoid pleural effusions complicated by Mycobacterium heckeshornense infection. Respir Med Case Rep. 2018;25:333-335. https://doi.org/10.1016/j.rmcr.2018.10.021.
19. Kinoshita H, Ono M, Nagatomo Y, et al. Diagnosis of pulmonary Mycobacterium heckeshornense infection using matrix-assisted laser desorption ionization time-of-flight mass spectroscopy. IDCases. 2021;26:e01296. https://doi.org/10.1016/j.idcr.2021.e01296.
Dr Werry is a clinical pharmacy specialist, infectious diseases, at Surrey Memorial Hospital and a clinical instructor in the Faculty of Pharmaceutical Sciences at the University of British Columbia. Dr Eshtiaghi is a resident physician in the Division of Dermatology in the Department of Medicine at the University of Toronto. Dr Trippell is a resident physician in the Department of Family Practice at UBC. Dr Masud is a medical microbiologist at Surrey Memorial Hospital and a clinical assistant professor in the Department of Pathology and Laboratory Medicine at UBC. Dr Sekirov is a medical microbiologist at the BC Centre for Disease Control Public Health Laboratory and a clinical assistant professor in the Department of Pathology and Laboratory Medicine at UBC. Dr Mirzanejad is a consultant, infectious diseases and tropical medicine, at Surrey Memorial Hospital and a clinical professor in the Division of Infectious Diseases in the Department of Medicine at UBC. Dr Wong is head of the Department of Medicine at Surrey Memorial Hospital and a clinical associate professor in the Division of Infectious Diseases in the Department of Medicine at UBC.
Corresponding author: Dr Patrick H.P. Wong, patricke@mail.ubc.ca.