Original Research
Cutting it close: Five-year retrospective review of melanoma guideline adherence in Northern British Columbia
ABSTRACT
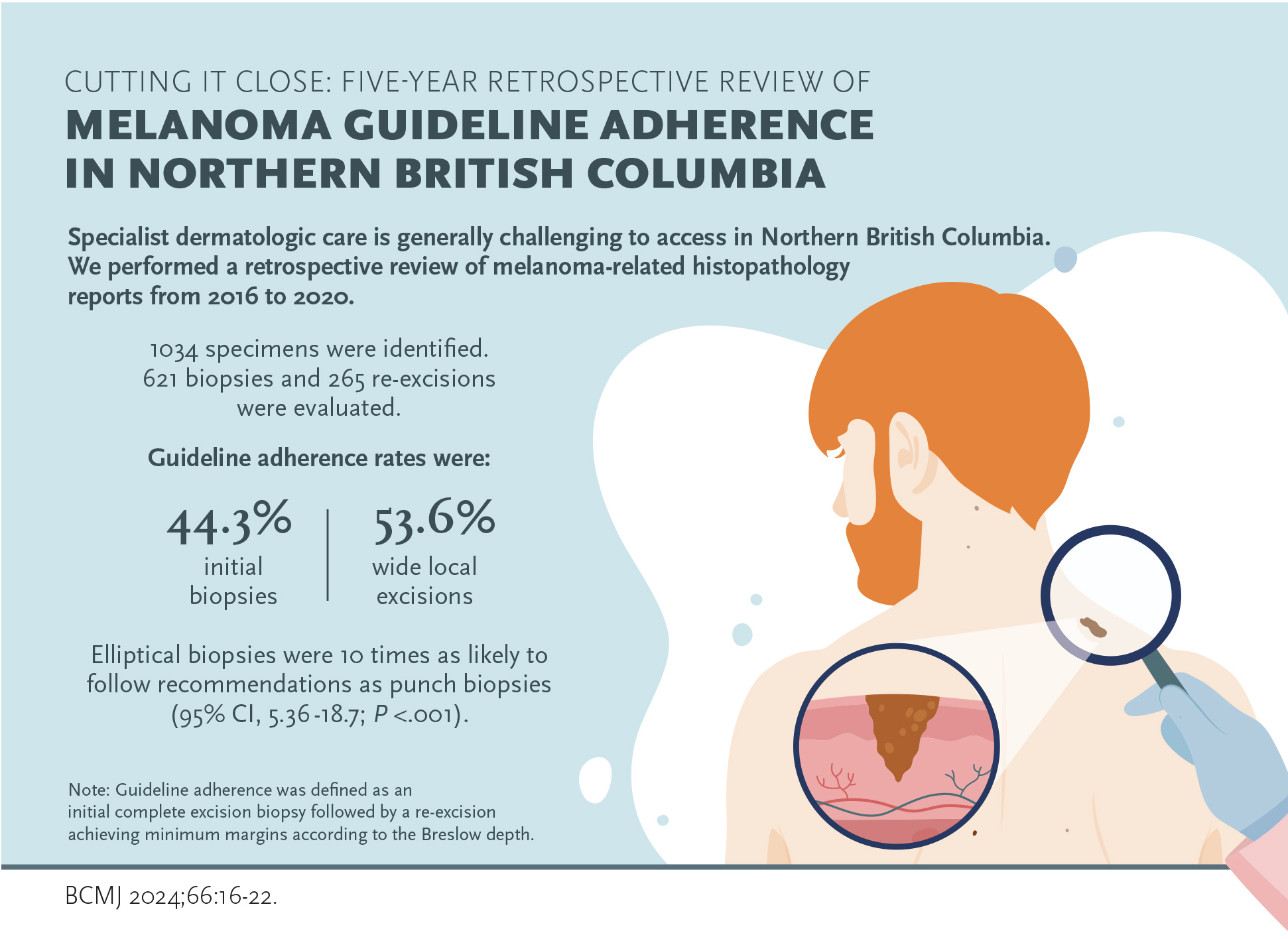
Background: Specialist dermatologic care is generally challenging to access in Northern British Columbia. We aimed to quantify guideline adherence rates in the Northern Health Region for both biopsies of suspected melanoma and wide local excisions of confirmed melanoma.
Methods: We performed a retrospective review of melanoma-related histopathology reports from 2016 to 2020. Guideline adherence was defined as an initial complete excision biopsy followed by a re-excision achieving minimum margins according to the Breslow depth.
Results: Of 1034 specimens identified, we evaluated 621 biopsies and 265 re-excisions. Guideline adherence rates were 44.3% for initial biopsies and 53.6% for wide local excisions. Elliptical biopsies were 10 times as likely to follow recommendations than punch biopsies (95% CI, 5.36-18.7; P < .001).
Conclusions: Multiple deviations from published standards were identified in the clinical management and pathology reports. A melanoma database and standardized reporting are needed to fully evaluate current practices in Northern British Columbia.
Primary cutaneous melanoma is a potentially life-threatening disease treated primarily by surgical excision. Adherence to expert consensus guidelines likely helps physicians optimize patient outcomes.
Introduction
Primary cutaneous melanoma is a potentially life-threatening disease treated primarily by surgical excision. Guidelines recommending complete excisional biopsies with clinical margins have been published by the American, Australian, and British dermatological associations.[1-3] The initial biopsy is vital for staging melanomas via the Breslow depth and ulceration status, which determine the re-excision margins. See Table 1 for stage-specific wide local excision margins.
While BC Cancer does not provide specific guidelines for the initial excision, the American Academy of Dermatology recommends a narrow excisional/complete biopsy with 1–3 mm margins that encompasses the entire breadth of the lesion and is of sufficient depth to prevent transection at the base.[1] This can be accomplished by elliptical excision, punch excision around the entire lesion, or deep shave/saucerization to a depth below the lesion. A clinical example is shown in Figure 1.
If a suspected melanoma is only partially excised, the thickest part of the melanoma may be missed, which risks underestimating the Breslow depth [Figure 2]. According to clinical practice guidelines, incisional biopsies should be performed only in sensitive or cosmetically important areas.[1,2,4,5] However, the margins of the subsequent wide local excision should follow current guidelines, regardless of location, unless the risks of underexcising are clearly discussed with the patient [Figure 3].
Incisional biopsy of a melanoma alone does not appear to directly affect the local recurrence or overall survival of melanoma patients.[6] However, a 2014 study found a significant proportion of Breslow depth values upstaged at the subsequent wide local excision when the initial biopsy was incisional.[7] Incisional biopsies often underestimate the Breslow depth, potentially leading to subsequent wide local excision margins that are too narrow for the true tumor stage. Additionally, incisional sampling prevents assessment of vital histopathologic features in the differentiation of melanoma from nevus, including breadth, symmetry, and, to some extent, circumscription. Thus, a partial excision may miss the diagnosis of melanoma altogether; this rate has been measured at 23% for punch biopsies.[8] Taken together, adherence to expert consensus guidelines likely helps physicians optimize patient outcomes.
British Columbia’s Northern Health Region primarily serves rural and remote populations. Practitioners in Northern BC have historically had challenges accessing specialist care. Given this, we raised the question of whether this impacted guideline adherence in the region for melanoma biopsy and re-excision. To our knowledge, there has never been an audit of melanoma surgeries within the Northern Health Region. This study aimed to quantify guideline adherence rates for both initial biopsies of suspected melanomas and final melanoma re-excisions within the region.
Materials and methods
We conducted a retrospective chart review of histopathology reports for all melanoma-related cases in the Northern Health Region from 2016 to 2020. Ethics approval was granted by the Northern Health Research Review Committee. All histopathology reports containing the word “melanoma” were identified. Ordering physician and patient demographic data were removed. Each patient and physician was given a unique identifying number. Operational variables were pilot tested with 2016 data; one author abstracted the final data. Lesions were not selected based on histopathologic diagnosis; thus, biopsies of both benign and malignant lesions were included. For re-excisions, all primary cutaneous invasive melanoma and melanoma in situ were included. Relevant patient history was extracted from the requisition when available. Peripheral and deep histologic margin distance was recorded as the nearest approach to either invasive or in situ melanoma. The face, scalp, ears, hands, feet, nipples, and anogenital areas were defined as sensitive locations. Exclusion criteria included irrelevance to melanoma, conjunctival and ungual specimens, and atypical specimens such as amputations.
Adherence to management guidelines was compared with the recommendations available on the BC Cancer website.[9] For biopsies, adherence to guidelines was defined as achieving complete excision (determined by gross margins) and inclusion of at least the superficial dermis. In cases where clinical margins were unavailable, a minimum biopsy width of 6 mm was set, given that the majority of melanomas have a diameter greater than 6 mm.[10,11] In cases where gross margins were unknown, they were inferred from the histologic margins or substituted by the stated clinical margins in the requisition. Due to an incomplete data set, these uncertain data were included separately in select analyses as either likely adherent or likely nonadherent.
Re-excision gross margins were counterchecked with prior biopsy Breslow depth to assess adherence to respective minimum excisional margins [Table 1]. If gross margins were unavailable, they were approximated by halving the maximum width of the specimen. Gross measurements were allotted error margins of 21% for length and 12% for width to adjust for sample shrinkage.[12] When margins were equal to or extremely near the guideline standard (e.g., < 0.1 mm margins or 2.0 cm re-excision margins), they were categorized as borderline and separated in the analyses.
Initial data were inspected using univariate analyses, including contingency tables and descriptive statistics. The mean and standard deviation were used to summarize continuous data. Categorical data were analyzed with the chi-square test, and continuous variables with the Student’s t test. A two-tailed P value of ≤ .05 was considered statistically significant.
Results
Of the 1034 specimens identified during the study period, 31 were excluded, including 18 irrelevant cases and 13 other specimens, such as frozen sections and subungual biopsies. We collected 18 core needle biopsies, 13 lymphadenectomies, 56 sentinel lymph node biopsies, 17 subcutaneous masses, and 15 internal metastasis specimens. Overall, 886 cutaneous specimens formed the data set, composed of 621 biopsies of pigmented lesions (any pathologic diagnosis) and 265 re-excisions (pathology-confirmed melanoma, invasive and in situ).
Cutaneous biopsies
In 46 (7.4%) skin biopsies, the biopsy technique (e.g., punch) was not mentioned in the pathology requisition. After excluding these and two from unknown anatomic locations, data from the remaining 573 cases were analyzed [Table 2]. The primary techniques employed for biopsies were elliptical (61.6%), punch (30.9%), and shave (3.3%). Nontraditional formats were used in 4.2% of biopsies, including saucerization, wedge, narrow incisional fusiform, multiple punch biopsies within the same lesion, and placing multiple lesions in the same formalin container. On 16 instances, a second biopsy was taken as an intermediate step between an incisional biopsy and wide local excision.
Minimal clinical history was provided in most pathology requisitions and rarely mentioned the suspected diagnosis or whether the biopsy was incisional or excisional, which can inform tissue sectioning technique. Anatomic locations were not provided for two surgical specimens and four sentinel lymph node biopsies.
Incisional biopsies were slightly more frequent in sensitive anatomical areas. Excisional biopsies were taken with a 2.47 mm clinical margin on average (SD 2.45 mm, n = 118). Of all biopsies, 5.3% (14/265) were too shallow and had positive deep margins. Excluding these, the mean Breslow depth of incisional biopsies (1.68 mm) was lower than that of excisional biopsies (2.08 mm) but did not reach statistical significance.
Excluding those in cosmetically sensitive areas, 44.3% (159/359) of initial biopsies adhered to guidelines [Figure 4]. Elliptical biopsies were 10 times as likely to follow guidelines as punch biopsies (odds ratio 10.0; 95% CI, 5.36-18.7; P < .001). Reasons for not meeting guidelines were nonexclusive and distributed as follows: inadequate width (212), partial excision (83), shallow depth (6), multiple samples per lesion or container (14), and other technical errors (3), such as a nonperpendicular angle.
The occurrence rates of five important prognostic factors (ulceration, mitotic rate, satellitosis, lymphovascular invasion, and neurotropism) in biopsies positive for invasive melanoma are in Table 3. Ulceration status was not reported in 19.91% of melanoma biopsy reports. Approximately half (116/226) of invasive melanoma biopsy reports reported all five prognostic factors.
In 5.9% of biopsies, the Breslow value could not be determined due to melanoma extending to the deep margin. Nonmetastatic melanomas biopsied with negative deep margins had a mean Breslow depth of 1.85 mm (SD 2.26 mm, range 0.08–12.0 mm, n = 175).
Wide local excisions
There were 265 wide local excisions, including 28 repeat attempts (third surgery following a biopsy and re-excision with inadequate margins). There were 27.1% fewer re-excisions than expected, since all positive biopsies should theoretically prompt a re-excision. The disproportionate number of re-excisions suggests that not all biopsy-proven melanomas were followed up with a wide local excision. However, there is a possibility that some of these patients died or traveled out of the area for their re-excisions.
Of wide local excisions with available measurements, 53.6% (103/192) adhered to guidelines [Figure 5]. Re-excision guideline adherence decreased to 41.0% (16/39) in sensitive body locations. Deviations from published guidelines were primarily in the way of inadequate surgical margins. However, in three instances, providers aimed only to re-excise a specimen with a 5 mm total width, rather than re-excising 5 mm of healthy tissue on both sides of the scar. Residual tumor was present in 30.5% (81/265) of all re-excisions. Four re-excisions had positive gross margins, and 6.3% (16/253) presented with positive histologic margins.
Discussion
This 5-year melanoma retrospective review in the Northern Health Region in British Columbia identified multiple areas where melanoma guidelines were not adhered to. Across anatomic sites, approximately one-third of biopsies for suspected melanoma were incisional. Even excluding those in cosmetically sensitive areas, only 44.3% of initial biopsies adhered to guidelines. For wide local excisions, guideline adherence was slightly higher, at 53.6%.
Overall, less than half of surgical excision specimens followed BC Cancer recommendations. Adherence rates were lower than those in other countries, which ranged from 88% to 96.8%.[13,14] Rural populations with a low geographical density of dermatologists have previously been found to have a greater proportion of melanomas with Breslow depths greater than 2 mm.[15] Another study showed that melanoma guidelines were less likely to be followed in rural areas than urban areas.[16]
Significant barriers exist to the uptake of melanoma guidelines. Smaller biopsies may be more comfortable for physicians who do not regularly perform surgical procedures. Time constraints, remuneration, and cosmesis may also play a role. Some argue that strict guidelines pose barriers to biopsy for physicians, resulting in delayed or fewer diagnostic biopsies. There is also no evidence that incisional biopsies adversely affect patient outcomes, if one assumes that incisional biopsies do not miss some melanomas altogether.[8] Given current evidence and the potential consequences of incompletely excising melanomas, we recommend that physicians continue to strive to meet BC Cancer guidelines. Melanoma guidelines were developed through expert consensus and interpretation of available evidence to standardize the clinical management of melanoma and improve quality of care. Whether adherence to recommended margins plays a major factor in long-term survival is controversial and the subject of other studies.
When submitting a sample, providing more complete clinical information can be helpful for the pathologist. Stating the lesion’s clinical size, the biopsy intent, and the area sampled is important. The excisional versus incisional status of the biopsy can impact tissue preparation and aid pathologists in the diagnostic process. If an incisional biopsy is clinically indicated due to a large lesion or a cosmetically sensitive location, sampling with a scalpel or punch biopsy in the most atypical part of the lesion is appropriate.
Future directions
Further surgical training may lead practitioners to feel more confident to follow excision guidelines. We encourage practitioners to review the most current guidelines before melanoma-related procedures. We recommend that most wide local excisions be referred to practitioners who are comfortable undertaking surgical re-excisions and regularly do so. To ensure high standards of melanoma care, interest is increasing in determining and monitoring the quality of interventions. A prospective study is needed to better assess clinical melanoma management in the Northern Health Region. Standardizing melanoma reports and creating a melanoma data registry are needed to fully evaluate current practices. Promoting standardization and quality assurance of surgical procedures is likely to improve patient outcomes.[17] Pathology reports can help emphasize guidelines—for instance, by explicitly stating when clinical history was missing, that a biopsy was incisional, or next steps in management (e.g., “Although the biopsy margins are negative for melanoma, a 10 mm re-excision +/− sentinel lymph node biopsy is indicated”). These study results can inform targeted educational initiatives in the region.
Limitations
This study had significant limitations. Data were coded by a single nonblinded author. Since pathology requisitions rarely included clinical differential diagnoses, some biopsies of nonmelanocytic lesions without suspicion for melanoma may have been included and inappropriately held to melanoma biopsy standards. Some biopsies occurred outside of the Northern Health Region or the study time frame; thus, re-excision target margins could not be verified. These factors resulted in a largely incomplete data set. Margin clearance and prognostic factors were more frequently reported in aggressive melanomas, skewing the data toward advanced disease. Last, shave biopsies and small punches are inaccurate representations of tumor distance from margins due to specimens stretching during slide preparation.
Conclusions
In this study, guidelines for melanoma-related biopsies and wide local excisions in Northern British Columbia were often not adhered to. Whether guidelines impact long-term patient outcomes is controversial, but considering the stakes of missing a melanoma, striving for guideline-based care for melanoma patients remains important. Changes can be made in multiple dimensions—from biopsy to pathology report to subsequent re-excision—and these results can be used to inform physician education in the future. A prospective study should be undertaken to better document guideline adherence rates and the effect on patient outcomes.
Competing interests
None declared.
Funding
The authors received no financial support for the research, authorship, or publication of this article.
Data availability statement
The data that support the findings of this study are available upon request from the corresponding author, C.S. The data are not publicly available, as they contain details that could compromise patient privacy.
Acknowledgments
The authors would like to thank Dr Doug Sawyer for reviewing the article and providing dermatopathology expertise. They also wish to acknowledge Dr Taylor Callander for contributing to the study design and requesting institutional approval.
Clinical takeaways
- Punch biopsies should encompass the entire lesion, unless in a sensitive anatomic location. In this study, elliptical biopsies were 10 times as likely to follow recommendations as punch biopsies.
- Measure re-excision margins from the perimeter of the biopsy scar. If re-excising 5 mm margins, the total width of the specimen should be a minimum of 10 mm.
- In cosmetically sensitive areas or with a large lesion, an incisional biopsy may be clinically indicated. Sampling with a scalpel or punch biopsy within the most atypical part of the lesion is appropriate.
- Include the following elements in a pathology requisition:
- Site, description, and size of lesion.
- Excisional or incisional intent.
- Type of biopsy (e.g., ellipse, punch, shave).
Key terms
Breslow depth: Maximum tumor thickness, measured from the top of the granular layer of the epidermis (or the base of an ulcer) to the deepest point of tumor involvement.
Invasive melanoma: Melanoma present in the dermis or deeper.
Melanoma in situ: Melanoma confined to the epidermis.
Melanoma staging: Based on tumor thickness (T), lymph node involvement (N), and presence of distant metastases (M). See Table 1 for tumor staging details and corresponding re-excision margins.
Sentinel lymph node biopsy: Identification and excision of the first lymph node(s) the primary melanoma tumor is most likely to spread to. This procedure is considered for stage T1b and recommended for stage T2a and higher.[1] A positive sentinel lymph node biopsy leads to consideration for lymphadenectomy and adjuvant therapy.
This article has been peer reviewed.
 |
| This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. |
References
1. Swetter SM, Tsao H, Bichakjian CK, et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol 2019;80:208-250.
2. Sladden MJ, Nieweg OE, Howle J, et al. Updated evidence-based clinical practice guidelines for the diagnosis and management of melanoma: Definitive excision margins for primary cutaneous melanoma. Med J Aust 2018;208:137-142.
3. Hanna S, Lo SN, Saw RP. Surgical excision margins in primary cutaneous melanoma: A systematic review and meta-analysis. Eur J Surg Oncol 2021;47:1558-1574.
4. Marsden JR, Newton-Bishop JA, Burrows L, et al. Revised U.K. guidelines for the management of cutaneous melanoma 2010. Br J Dermatol 2010;163:238-256.
5. Garbe C, Amaral T, Peris K, et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics—Update 2019. Eur J Cancer 2020;126:141-158.
6. Bong JL, Herd RM, Hunter JAA. Incisional biopsy and melanoma prognosis. J Am Acad Dermatol 2002;46:690-694.
7. Kaiser S, Vassell R, Pinckney RG, et al. Clinical impact of biopsy method on the quality of surgical management in melanoma. J Surg Oncol 2014;109:775-779.
8. Ng JC, Swain S, Dowling JP, et al. The impact of partial biopsy on histopathologic diagnosis of cutaneous melanoma: Experience of an Australian tertiary referral service. Arch Dermatol 2010;146:234-239.
9. BC Cancer. Melanoma. In: Cancer management manual. Accessed 4 November 2023. www.bccancer.bc.ca/health-professionals/clinical-resources/cancer-management-manual/skin/melanoma.
10. Helsing P, Loeb M. Small diameter melanoma: A follow-up of the Norwegian Melanoma Project. Br J Dermatol 2004;151:1081-1083.
11. Soltani-Arabshahi R, Sweeney C, Jones B, et al. Predictive value of biopsy specimens suspicious for melanoma: Support for 6-mm criterion in the ABCD rule. J Am Acad Dermatol 2015;72:412-418.
12. Kerns MJJ, Darst MA, Olsen TG, et al. Shrinkage of cutaneous specimens: Formalin or other factors involved? J Cutan Pathol 2008;35:1093-1096.
13. Livingstone E, Windemuth-Kieselbach C, Eigentler TK, et al. A first prospective population-based analysis investigating the actual practice of melanoma diagnosis, treatment and follow-up. Eur J Cancer 2011;47:1977-1989.
14. Blakely AM, Comissiong DS, Vezeridis MP, Miner TJ. Suboptimal compliance with national comprehensive cancer network melanoma guidelines: Who is at risk? Am J Clin Oncol 2018;41:754-759.
15. Barbe C, Hibon E, Vitry F, et al. Clinical and pathological characteristics of melanoma: A population-based study in a French regional population. J Eur Acad Dermatol Venereol 2012;26:159-164.
16. Green J, Murchie P, Lee AJ. Does patients’ place of residence affect the type of physician performing primary excision of cutaneous melanoma in Northern Scotland? J Rural Health 2013;29(Suppl 1):35-42.
17. Pasquali S, Sommariva A, Spillane AJ, et al. Measuring the quality of melanoma surgery—Highlighting issues with standardization and quality assurance of care in surgical oncology. Eur J Surg Oncol 2017;43:561-571.
Dr Doyon earned her medical degree from the University of British Columbia and is a first-year dermatology resident in the Division of Dermatology, Centre Hospitalier de l’Université de Montréal. Dr Lindenbach is a second-year family medicine resident in the Department of Family Practice, UBC. Dr Heydarzadeh-Azar is a staff pathologist in Fort St. John and a clinical instructor in the Department of Pathology and Laboratory Medicine, UBC. Dr Sladden is a community dermatologist in Courtenay and Dawson Creek and a clinical instructor in the Department of Dermatology and Skin Science, UBC.


I've done both excisional and incisional biopsies for pigmented lesions. There are many practical reasons why an incision might be done, knowing full well there may be an underestimation of depth due to sampling error. The rationale for punch biopsying a large lesion is practical - if atypia or melanoma is found, referral to plastics or dermatology for resection with adequate wide margins, with potential grafting, electrocautery, anesthesia can all contribute to this being a better approach.