Antibiotic treatment durations are not based on scientific reasoning; they stem from an artificially created number system
ABSTRACT: Antibiotics are often prescribed for longer than necessary, and treatment durations are arbitrary and inherently flawed. With the ongoing global threat of antimicrobial resistance, reducing treatment length to the minimum effective duration is a priority. However, achieving an optimum duration is not an easy feat, because the scientific underpinnings of modern antibiotic durations are weak or nonexistent.
A critical look at the evidentiary basis of our modern antibiotic treatment lengths.
As an infectious diseases physician, I am often asked, “How long do I treat this patient’s infection?” After years of extensive medical training, clinical practice, and memorization of treatment durations from various infectious diseases guidelines, surely my answer should be based on credible scientific evidence, right? There is a plethora of randomized controlled trials comparing different treatment durations for many types of infections, including streptococcal pharyngitis,[1] community-acquired pneumonia,[2] and gram-negative bacteremia,[3] to name a few. However, is the method by which antibiotic treatment durations are selected based on scientific rationale or on a convenient and pleasing system of numbers? In this article, I take a critical look at the evidentiary basis of our modern antibiotic treatment lengths.
How to determine duration of treatment
On 16 October 2010, Dr Daniel Gilbert, a professor of psychology at Harvard University, published an op-ed in The New York Times describing his encounter at a local emergency department.[4] He received a 7-day course of antibiotics for a minor ailment. As a psychologist, he rightly posed the question: “What I didn’t understand was why a full course took precisely seven days. Why not six, eight or nine and a half? Did the number seven correspond to some biological fact about the human digestive tract or the life cycle of bacteria?”
On 22 October 2010, Dr Paul Sax, an infectious diseases physician and an editor for the New England Journal of Medicine Journal Watch, responded satirically to Dr Gilbert’s question: “The answer, Dr Gilbert, is that this is highly-specialized knowledge, rarefied information that only 100% Board-Certified, USDA-inspected Infectious Diseases Doctors know. And since I’m concerned that your article might give readers the wrong impression about our scientific credibility, I’ll now divulge what we’ve learned, and how to apply it.”[5] Dr Sax then wittily revealed the seven golden rules for choosing an antibiotic duration:
- Choose a multiple of 5 (fingers of the hand) or 7 (days of the week).
- Is it an outpatient problem that is relatively mild? If so, choose something less than 10 days. After application of our multiples rule, this should be 5 or 7 days.
- Is it really mild, so much so that antibiotics probably aren’t needed at all but clinician or patient are insistent? Break the 5/7 rule and go with 3 days. Ditto uncomplicated cystitis in young women.
- Is it a serious problem that occurs in the hospital or could end up leading to hospitalization? With the exception of community-acquired pneumonia (5 or 7 days), 10 days is the minimum.
- Patient not doing better at the end of some course of therapy? Extend treatment, again using a multiple of 5 or 7 days.
- Does the infection involve a bone or a heart valve? Four weeks (28 days) at least, often 6 weeks (42 days). Note that 5 weeks (35 days) is not an option—here the 5s and 7s cancel each other out, and chaos ensues.
- The following lengths of therapy are inherently weird, and should generally be avoided: 2, 4, 6, 8, 9, 11, 12, 13 days. Also, 3.14159265 days.[5]
The takeaway from this comical exchange is that treatment durations are mostly arbitrary. Consider the following sequence of numbers, and fill in the blank: 2, 3, 5, 7, X, 13, and 17. The only value that makes mathematical sense is 11, as all are prime numbers. Now consider antibiotic treatment durations in these two sequences: 3, 5, 7, X, and 14 days and 1, 2, X, and 6 weeks. What goes in the blanks? Most of us would likely answer 10 days and 4 weeks, but why? Do these number sequences serve a mathematical or scientific purpose? Do they have a special meaning or magical powers? In 321 AD, the Roman emperor Constantine the Great declared that there would be 7 days in a week.[6] As humans, we have 5 digits per limb. Hence, we often choose durations that are multiples of 5 or 7 for convenience. To highlight the silliness of this prescribing pattern, one expert coined the term “Constantine unit.”[6]
According to psychologists, humans have a propensity toward magical thinking that develops in childhood but can persist into adulthood.[7] The application of magical thinking to numbers is called numerology—the belief in the mystical and divine relationship between numbers and outcomes. Consequently, our preferences for particular treatment durations are based not on science, but rather on the way we organize numbers in our minds. We think in chunks of time based on arbitrary human constructs. In my experience, a particularly potent number is 6 weeks, as many complicated infections that demand prolonged antibiotic therapy are often treated with a 6-week course in the absence of guiding evidence. Is it logical to assume that biology, physiology, or microbes care about our arbitrary units of time?
Since our unit of time is based on the 24-hour rotational cycle of the Earth, I wonder how our treatment durations would be different if humans had evolved 2.5 billion years ago, when it took only 18 hours for the Earth to make a complete rotation about its axis. If humans were to ever become an interplanetary species, would we need to recalibrate our treatment durations to correspond to the rotational speed of the planet we lived on? This would be problematic, as some planets spin very slowly (1 Venus day = 243 Earth days), while others spin faster (1 Jupiter day = 0.42 Earth days).[8]
In clinical trials comparing two different antibiotic durations for certain infections, the short and long treatment arms are typically multiples of 5 and 7, respectively, even though study investigators can choose any numbers they desire.[9-11] The fixation on these numbers can be quite strong, potentially limiting us from prescribing durations that might be better for some patients. Note that if noninferiority of the short duration is demonstrated, it does not mean the short treatment is the optimal duration, as optimal duration varies from person to person. It would be unwise to assume a fixed duration of treatment applies to every patient, because differences in host and microbial factors affect the time to recovery. How, then, do we determine the correct duration of antibiotic to prescribe?
How to determine when the patient is cured
The optimal treatment duration is the minimum number of days, weeks, or months of antibiotic required to cure the patient of their infection. We typically rely on clinical, biochemical, microbiological, and/or radiographic findings to determine when the patient has been adequately treated for their infection. However, these parameters do not necessarily tell us that tissue destruction/invasion by the pathogen has ceased. It is common for clinical, biochemical, and radiographic abnormalities to persist beyond the completion of the antibiotic course, because the healing process (e.g., tissue repair, immune downregulation, symptom resolution) can last much longer.[12,13] In practice and in clinical trials, the definition of cure is arbitrary and can include as many or as few clinical, biochemical, microbiological, and radiographic elements as one desires. The greater the number of criteria required or the more stringent they are to satisfy the definition of cure, the harder it is for patients to achieve it. From a microbiological standpoint, cure is achieved when pathogens are no longer able to invade and multiply.
The goal of the antibiotic is to kill the pathogens or render them incapable of causing further disease. How much of the microbial inoculum do you need to kill to achieve this goal—80%, 90%, 100%? Do we even know? The vast majority of patients have an intact immune system that can fight off the invading microbes too, so we are not relying on the drug to do all the work. How do you determine when enough of the pathogens have been killed off that you can stop treatment? Unfortunately, we don’t yet know how to figure this out. This realization calls into question our fundamental understanding of what it means to be cured. Persistent symptoms and signs of inflammation do not necessarily indicate ongoing infection.[12,13] On the other hand, the absence of inflammatory symptoms and signs does not exclude infection either.[14,15] Moreover, patient symptoms correlate poorly with pathogen burden.[16] Therefore, we don’t actually know when a patient is truly cured of their infection, and, accordingly, an optimal duration might simply be an illusion.
Treatment durations in practice
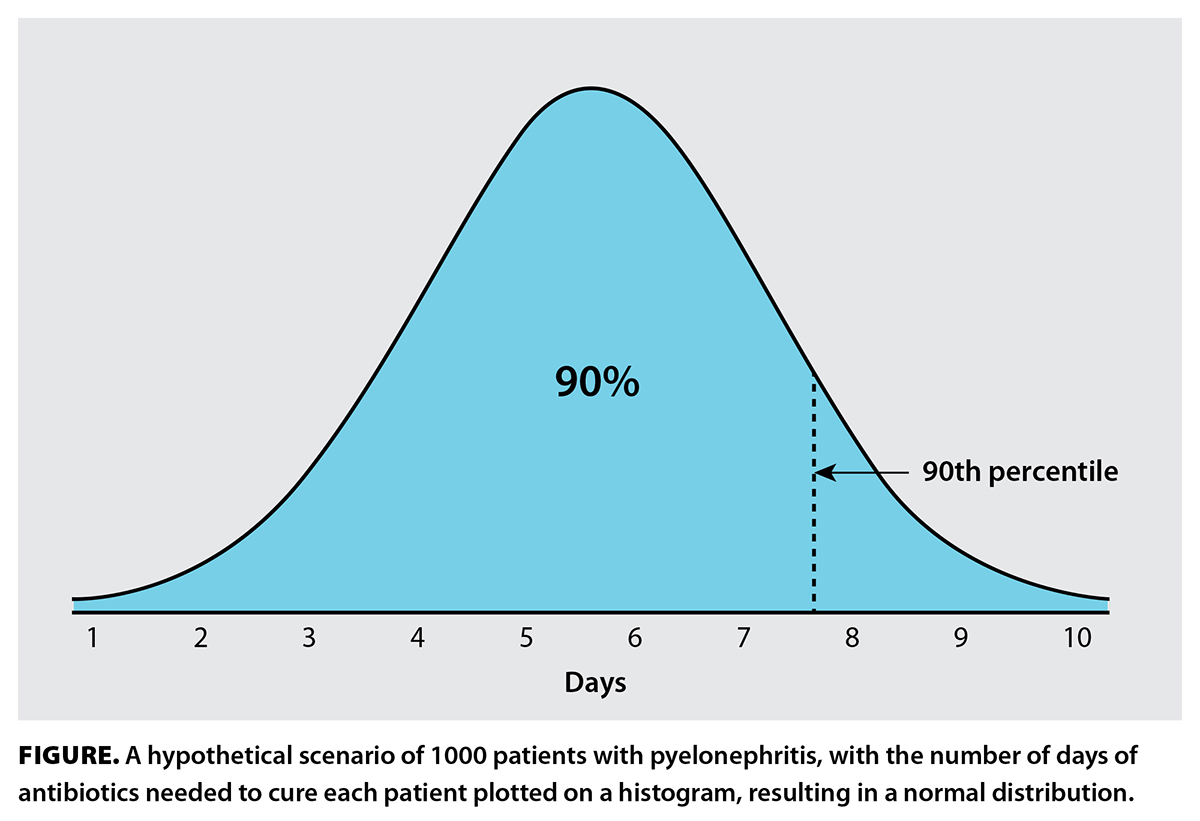 Clinicians tend to favor certain treatment durations, particularly 5, 7, 10, and 14 days.[17] These fixed durations are inadequate, as they are not individualized. Consider this hypothetical scenario: There are 1000 patients with pyelonephritis, and you know exactly how many days of antibiotics are needed to cure each patient. You plot these numbers on a histogram, ending up with a normal distribution [Figure]. You are tasked with writing a recommendation on treatment duration in a guideline. Should you recommend 6 days (median)? That won’t work, because only half of the patients will be cured. How about 8 days, so that at least 90% are expected to be cured? Unfortunately, 8 days results in overtreatment of most patients, who require 7 or fewer days. How about a range, such as 1 to 10 days? This would seem to be the most correct answer, but it fails if the clinician is unable to determine the precise number of days required for their patient. To combat this problem, biomarkers such as C-reactive protein and procalcitonin can be used to guide antibiotic duration, but these tools have limited predictive power of when the infection will have cleared.[18] Additionally, validated clinical criteria can also be used to determine the length of therapy.[19] Treatment guided by biomarkers and clinical criteria are a step in the right direction toward personalized antibiotic durations.
Clinicians tend to favor certain treatment durations, particularly 5, 7, 10, and 14 days.[17] These fixed durations are inadequate, as they are not individualized. Consider this hypothetical scenario: There are 1000 patients with pyelonephritis, and you know exactly how many days of antibiotics are needed to cure each patient. You plot these numbers on a histogram, ending up with a normal distribution [Figure]. You are tasked with writing a recommendation on treatment duration in a guideline. Should you recommend 6 days (median)? That won’t work, because only half of the patients will be cured. How about 8 days, so that at least 90% are expected to be cured? Unfortunately, 8 days results in overtreatment of most patients, who require 7 or fewer days. How about a range, such as 1 to 10 days? This would seem to be the most correct answer, but it fails if the clinician is unable to determine the precise number of days required for their patient. To combat this problem, biomarkers such as C-reactive protein and procalcitonin can be used to guide antibiotic duration, but these tools have limited predictive power of when the infection will have cleared.[18] Additionally, validated clinical criteria can also be used to determine the length of therapy.[19] Treatment guided by biomarkers and clinical criteria are a step in the right direction toward personalized antibiotic durations.
Treatment durations are getting shorter
With the emergence of numerous randomized controlled trials demonstrating the noninferiority of shorter treatment durations, we now realize that many infections were historically treated for far too long.[20] It was previously thought that longer treatment was necessary to prevent infection relapse and subsequent development of antibiotic resistance, but modern clinical trials have repeatedly debunked this misconception.[21-23] Physicians tell their patients to finish a course of antibiotics even when they are feeling better, which stems from the aforementioned misconception. Considering that many patients receive antibiotic prescriptions for longer than the guideline-recommended duration and we do not yet know how to accurately individualize treatments, it is conceivable that this advice is never correct and may, in fact, be harmful. We are now recognizing that the opposite statement is true in some cases—stop the course of antibiotics when you are feeling better—but further research is needed to determine what feeling better means.[16,24] For example, in a clinical trial comparing 3 versus 8 days of antibiotics for the treatment of community-acquired pneumonia, patients who reached clinical stability (normal vital signs and mental status) by day 3 were randomized to either stopping treatment or continuing until day 8.[19] Those who stopped the antibiotic on day 3 fared just as well as those who stopped on day 8. Short-course therapy was commonplace in the 1940s, when antibiotic courses given for 1 to 4 days for the treatment of pneumonia were curative in most patients.[23] Eighty years later, modern evidence has proven that this is still true. Some obvious advantages of shorter treatment include reduced selection pressure that drives antimicrobial resistance, lower costs, diminished risk of Clostridioides difficile infection, and decreased risk of adverse drug effects. As we continue to experiment with shorter lengths of therapy, we inch closer to that coveted optimum duration.
Conclusions
Antibiotic treatment durations do not appear to have any biological basis. Although fixed treatment lengths dominate the therapeutic paradigm of infection management, they are inadequate and can result in overtreatment of many patients. With advancements in medical technology, there is hope that treatment durations can be fine-tuned to increase precision in our therapeutic decisions. Let’s stop fixating on fixed durations and embrace the entire spectrum of numerals.
Competing interests
None declared.
This article has been peer reviewed.
 |
| This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. |
References
1. Holm AE, Llor C, Bjerrum L, Cordoba G. Short- vs. long-course antibiotic treatment for acute streptococcal pharyngitis: Systematic review and meta-analysis of randomized controlled trials. Antibiotics (Basel) 2020;9:733. https://doi.org/10.3390/antibiotics9110733.
2. Li Q, Zhou Q, Florez ID, et al. Short-course vs long-course antibiotic therapy for children with nonsevere community-acquired pneumonia: A systematic review and meta-analysis. JAMA Pediatr 2022;176:1199-1207. https://doi.org/10.1001/jamapediatrics.2022.4123.
3. Turjeman A, von Dach E, Molina J, et al. Duration of antibiotic treatment for Gram-negative bacteremia—Systematic review and individual participant data (IPD) meta-analysis. EClinicalMedicine 2022;55:101750. https://doi.org/10.1016/j.eclinm.2022.101750.
4. Gilbert D. Magic by numbers. New York Times. 16 October 2010. Accessed 20 February 2025. www.nytimes.com/2010/10/17/opinion/17gilbert.html.
5. Sax PE. How to figure out the length of antibiotic therapy. NEJM Journal Watch. 22 October 2010. Accessed 20 February 2025. https://blogs.jwatch.org/hiv-id-observations/index.php/how-to-figure-out-the-length-of-antibiotic-therapy/2010/10/22/.
6. Wald-Dickler N, Spellberg B. Short-course antibiotic therapy—Replacing Constantine units with “shorter is better.” Clin Infect Dis 2019;69:1476-1479. https://doi.org/10.1093/cid/ciy1134.
7. Brashier NM, Multhaup KS. Magical thinking decreases across adulthood. Psychol Aging 2017;32:681-688. https://doi.org/10.1037/pag0000208.
8. NASA Science. How long is one day on other planets? Accessed 22 February 2025. https://spaceplace.nasa.gov/days/en/.
9. Daneman N, Rishu A, Pinto R, et al. Antibiotic treatment for 7 versus 14 days in patients with bloodstream infections. N Engl J Med 2025;392:1065-1078. https://doi.org/10.1056/NEJMoa2404991.
10. Williams DJ, Creech CB, Walter EB, et al. Short- vs standard-course outpatient antibiotic therapy for community-acquired pneumonia in children: The SCOUT-CAP randomized clinical trial. JAMA Pediatr 2022;176:253-261. https://doi.org/10.1001/jamapediatrics.2021.5547.
11. Gjika E, Beaulieu JY, Vakalopoulos K, et al. Two weeks versus four weeks of antibiotic therapy after surgical drainage for native joint bacterial arthritis: A prospective, randomised, non-inferiority trial. Ann Rheum Dis 2019;78:1114-1121. https://doi.org/10.1136/annrheumdis-2019-215116.
12. Bruun T, Oppegaard O, Hufthammer KO, et al. Early response in cellulitis: A prospective study of dynamics and predictors. Clin Infect Dis 2016;63:1034-1041. https://doi.org/10.1093/cid/ciw463.
13. Chadda KR, Blakey EE, Davies TW, Puthucheary Z. Risk factors, biomarkers, and mechanisms for persistent inflammation, immunosuppression, and catabolism syndrome (PICS): A systematic review and meta-analysis. Br J Anaesth 2024;133:538-549. https://doi.org/10.1016/j.bja.2024.03.038.
14. Wake RM, Molloy SF, Jarvis JN, et al. Cryptococcal antigenemia in advanced human immunodeficiency virus disease: Pathophysiology, epidemiology, and clinical implications. Clin Infect Dis 2023;76:764-770. https://doi.org/10.1093/cid/ciac675.
15. Wang Y, Kang H, Liu X, Tong Z. Asymptomatic cases with SARS-CoV-2 infection. J Med Virol 2020;92:1401-1403. https://doi.org/10.1002/jmv.25990.
16. Borek AJ, Ledda A, Pouwels KB, et al. Stop antibiotics when you feel better? Opportunities, challenges and research directions. JAC Antimicrob Resist 2024;6:dlae147. https://doi.org/10.1093/jacamr/dlae147.
17. Mponponsuo K, Pinto R, Fowler R, et al. Fixed versus individualized treatment for five common bacterial infectious syndromes: A survey of the perspectives and practices of clinicians. JAC Antimicrob Resist 2023;5:dlad087. https://doi.org/10.1093/jacamr/dlad087.
18. Dark P, Hossain A, McAuley DF, et al. Biomarker-guided antibiotic duration for hospitalized patients with suspected sepsis: The ADAPT-sepsis randomized clinical trial. JAMA 2025;333:682-693. https://doi.org/10.1001/jama.2024.26458.
19. Dinh A, Ropers J, Duran C, et al. Discontinuing β-lactam treatment after 3 days for patients with community-acquired pneumonia in non-critical care wards (PTC): A double-blind, randomised, placebo-controlled, non-inferiority trial. Lancet 2021;397(10280):1195-1203. https://doi.org/10.1016/S0140-6736(21)00313-5.
20. Lee RA, Stripling JT, Spellberg B, Centor RM. Short-course antibiotics for common infections: What do we know and where do we go from here? Clin Microbiol Infect 2023;29:150-159. https://doi.org/10.1016/j.cmi.2022.08.024.
21. Spellberg B, Rice LB. Duration of antibiotic therapy: Shorter is better. Ann Intern Med 2019;171:210-211. https://doi.org/10.7326/M19-1509.
22. Spellberg B. The maturing antibiotic mantra: “Shorter is still better.” J Hosp Med 2018;13:361-362. https://doi.org/10.12788/jhm.2904.
23. Rice LB. The Maxwell Finland Lecture: For the duration-rational antibiotic administration in an era of antimicrobial resistance and Clostridium difficile. Clin Infect Dis 2008;46:491-496. https://doi.org/10.1086/526535.
24. Langford BJ, Morris AM. Is it time to stop counselling patients to “finish the course of antibiotics”? Can Pharm J (Ott) 2017;150:349-350. https://doi.org/10.1177/1715163517735549.
Dr Wong is a clinical assistant professor in the Division of Infectious Diseases, Department of Medicine, University of British Columbia, and an infectious diseases consultant at Royal Columbian Hospital and Eagle Ridge Hospital.
Corresponding author: Dr Davie Wong, davie.wong@fraserhealth.ca.

Very good and informative articles. Thank you for the important piece of information regarding the fact of duration of antibiotics.